Viability of Recycling Copper Indium Gallium Selenide (CIGS) in Photovoltaic Cells
This is a speculative class project and needs to be refined before being deployed at any scale.
Photovoltaics are devices which convert light energy into electrical energy. Photovoltaic (PV) cells can generate electricity for a wide range of electrical applications and can be implemented in most geographical locations and theoretically at any scale. A PV cell is only as good as the semi-conductor materials for which it is composed of. Specifically in this page we will be discussing CIGS as a semi-conductor material in a PV cell. According to the National Centre for Photovoltaic Research and Education (NCPRE), there are criteria we must evaluate of a semi-conductor material before we implement it in a design.[2] Those include:
- Absorption Coefficient: The higher the absorption coefficient the thinner the semi-conductor material can be. For thin film applications, this trait is preferred.
- Abundance of Raw materials: The semi-conductor materials used in any application must be readily available at a reasonable cost.
- Toxicity of material: The semi-conductor material must be non-hazardous to the environment.
- Stability of the material & junction: The chosen material for an application must be able to withstand the climate it is placed in and be chemically stable.
- Radiation resistance: Specifically speaking in most photovoltaic applications the semi-conductor material must not degrade to high energy radiation it may be exposed to.
To speak specifically about CIGS as an absorbing layer in PV cells we can note that:
CIGS in its chemical form is expressed as Cu(In,Ga)Se2, which belongs to the family of I-III-VI2 semiconducting materials that crystallize in a tetragonal chalcopyrite structure. Due to the high absorption coefficient and a direct band gap of 10^5 /cm, 1.04 eV - 1.70 eV respectively a very thin layer of CIGS is required perform the actions require in a PV cell. CIGS are also unique in that varying the Ga percentage the band gap can be tailored to be optimized in any situation. Specifically speaking we can tailor the band gap from 1.04 eV to 1.7 eV by changing the percent Ga. This unique ability of CIGS makes it an excellent material to be used in indoor applications (calculators etc.) along with large scale applications (solar fields, terrestrial etc.). The properties listed above; high absorption, flexible band gap (band gap equation see Eq. 1.1.1 below) allows to to compete with other semi-conductor materials. Such as; crystalline silicon, amorphous silicon and CdTe.[3]
For CuIn1-xGaxSe2 we can calculate the band gap as follows:
Eq. 1.1.1 Eg=1.010 + 0.626x - 0.167x(1-x)[3]
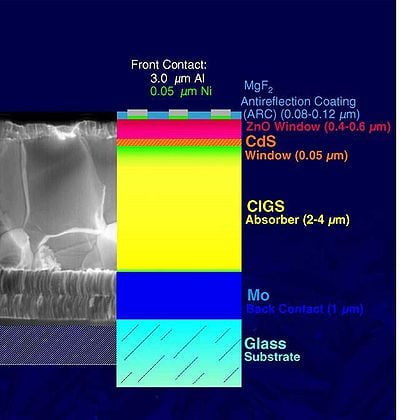
Aside from a high absorption coefficient and band gap flexibility, CIGS based solar cells are low cost to build and can be placed on cheaper substrates such as glass, metal or plastic. This is beneficial because CIGS does not require a high temperature for deposition. When constructing a CIGS based PV cell there are two possible models in which to follow; a Substrate Model or a Superstrate Model. See Figure 1 for a cross sectional view.
- Substrate Model: The back contact lies on a glass substrate, and light falls on solar cell top layer. For this extra passivation is required to avoid degradation of the solar cell.
- Superstrate Model:the front contact lies on a glass substrate and light falls from top of the surface. The benefit of this is it does not require any extra passivation.
Once a model is selected, the construction process of the CIGS based PV cell can begin. There are a variety of ways in which this can be done for either the Substrate or Superstrate models, including:
- Co-Evaporation[3]
- Here a three stage process is used.
- 1st stage:- Deposition of In, Ga and Se at relatively low temperature,
- 2nd stage:- Cu rich growth stage by evaporating Cu in excess at elevated temperature
- 3rd stage:- Deposits only In, Ga and Se to ensure the overall Indium (In) rich composition of the film.
- This construction process requires temperatures ranging from 450-500 degree Celsius.
- Selenization[3]
- In this construction process the first thermal process results in the deposition of Cu, In and Ga onto the Mo coated glass substrate. Once this process is complete the components undergo the selenization process which actually deposits the H2Se. Once after the second thermal process begins, which provides an absorber that is Cu(In,Ga)(S,Se)2 rather than the typical Cu(In,Ga)Se2 (which we showed above). The downfall of this method is that H2Se is very toxic requiring proper handling and care. Much like co-evaporation this process requires high substrate temperatures to promote the growth of CIGS phase.
- Spray Pyrolysis[3]
- Probably the least efficient method in regards to solar efficiency. In this method precursor films CuCl2, InCl3, SC(NH2)2 and GaCl3 are sprayed onto a preheated glass substrate at 350-400 degrees Celsius. Once precursor films are deposited annealing of the substrate is require, in environments such as H2Se at 450-500 degrees Celsius until the appropriate structure is obtained.
- Electrodeposition[3]
- This method relies on the principles of electrode potential between an electrochemical solution of Cu, In, Se and Ga. The differences in electrode potential between the elements in solution control when the atoms are deposited in the process. To induce deposition a system of three electrodes is used; a working electrode, counter electrode and a reference electrode. The working electrode and the counter electrode (also known as the inert electrode) are connected to a power source and induce the actual deposition. The reference electrode is necessary because it controls the potential of the working electrode to insure that a continuous and constant deposition is taking place on the substrate. Common materials for the reference electrode are Ag/AgCl and Gh/Hg2Cl2.
- Sputtering[3]
- The most "literal" process available. A substrate is heated to 400 degrees Celsius and placed into a vacuum around 10-4 torr. Once this has been established a power source connects the CIGS target and the substrate. This power source induces the sputtering.
Scale and Market for CIGS[edit | edit source]
While CIGS has good efficiencies for a thin film PV, it has been unable to overcome crystalline silicon PV. This is primarily due to an expensive manufacturing process and the economic downturn that significantly effected the photovoltaic sectors. With the recent capacity expansions and an increasing market for CIGS, investments are beginning to be made. NanoMarkets expects that CIGS produced for the PV market will grow from $613.4 million in 2011 to $5.41 billion in 2018. It is critical, however, for CIGS manufactures to develop less expensive processes and cheaper substrates in order to complete with conventional crystalline silicon in the PV market.[5]
The following outlines the current production processes for the most prevalent CIGS producing companies:
- Solibro - Co-evaporation CIGS process: Process which produces modules with a 17.4% power conversion efficiency[6][7]
- MiaSole - CIGS is deposited on a flexible stainless steel substrate entirely by continuous sputtering in a vacuum[8] to produce modules with an efficiency of 9-10%[9]
- Solyndra - Co-evaporation process in which Cu, In, Ga, Se are deposited straight onto glass tubes to produce modules with an efficiency of 7-10%[10]
- Global Solar - Co-evaporation CIGS process:[11] Uses an inline three stage deposition process[12] to produce modules with up to 19.9% efficiency in laboratory samples and 10.5%-11% average efficiency in production cells[13]
- Soltecture - Co-evaporation process[14] to produce modules with an efficiency up to 13% and an average efficiency of 12%[15]
- Nano Solar - Nanoparticle ink is used to print the semiconductor onto a flexible substrate[16] to produce modules with an efficiency of 8-9%[17]
NanoMarkets has identified three markets in which CIGS will be able to make a strong standing: the conventional panel market, rigid and flexible building integrated photovoltaics (BIPV), and the mobile market.
- Conventional Panel Market: A market that is becoming commoditized and competitive will require CIGS manufactures to develop manufacturing techniques and more efficient materials. At that point CIGS to potentially be sold at a price closer to crystalline silicon. CIGS manufacturers need to also consider residential and long term applications.
- Rigid and flexible BIPV: In this category CIGS have a significant advantage, specifically in the flexible BIPV sector. NanoMarkets now assumes that the majority of all flexible BIPV products will be constructed with CIGS. CIGS are very sensitive to moisture so to take advantage of the rigid BIPV market manufactures need to take advantage of BIPV lighter weight, direct deposition capabilities and higher power when compared directly to thin-film silicon.
- Portable Electronics: Portable systems require high power PV systems and CIGS can provide that. The market is not as large as the previous, conventional panel and BIPV, but the margins are expected to be quite large. CIGS manufactures would need to partner with consumer product companies to capture a vast majority of the market.
According to GTM Research, CIGS based PV cells shipments reached megawatt values. Specifically speaking we can see that the following companies produced the following values:[18]
- Solibro, 66 megawatts
- Miasole, 60 megawatts
- Solyndra, ~40 megawatts of production; number shipped is unknown
- Global Solar, 19 megawatts
- Soltecture, 14 megawatts
- Nano Solar, <10 megawatts
With the current market predictions as stated above, and with the technological advancement of CIGS manufacturing processes NanoMarkets assumes that revenue from CIGS will grow exponentially over the next 10 years.
Collection and Recycling Processes[edit | edit source]
Methods of Collecting the Lost Semiconductor Materials[edit | edit source]
The economic cost of landfill disposal is lower than the cost of recycling some PV modules.[19] This makes the idea of landfill disposal more favorable than recycling; however, there is a profit within recycling CIGS PV cells. In order to take advantage of this profit, the methods of collecting CIGS PV needs to be explored. The viability of collection for recycling is complicated, as there is a long interval (decades) between when the device is installed and when it is decommissioned. Additionally, there is a low concentration of valuable materials and a large geographical dispersion of CIGS PV modules. In order to accommodate all of these setbacks, three possible collection options of other products were presented by Fthenakis[20] and were applied to the recycling of CIGS PV modules.
- Utility Model: Large end-users (e.g., electric utilities) would be the primary owners and services of large PV systems and would therefore be responsible for collecting the end-of-life modules and delivering them to the recyclers. In this example, the recycling charges would be a part of the utility rates.
- Electronics Model: Manufacturers would be individually responsible for collecting the end-of-life modules and delivering them to the recyclers. Recycling in this case would most likely be done by integrated dismantlers and materials recyclers, and would be paid for by the generator, the manufacturer, or an escrow fund set aside at the time of purchase.
- Battery Model: Manufacturers would be collectively responsible for collecting the end-of-life modules and delivering them to recyclers. In this model, collection and transportation may be done by reverse retail channels and consolidation entities. The recycling would be completed by dismantlers and materials recyclers. Additionally, modules collected through reverse retail channels could be sent to smelters. In this example, recycling charges would be paid for by industry dues.
- Legal obligation: Since the Recast WEEE Directive came into force on August 13, 2012, collecting and recycling PV modules, including CIG(S), has become a mandatory requirement in the countries of the European Union[21] and hence needs to be added to the above listed models. While the WEEE Directive allows for both the collective (see Electronics Model) and individual (see Battery Model) concept, the Member States define – at national scale – the operational procedures.
Companies had begun to explore similar models too. Three developed and environmentally responsible recycling models were summarized by McDonald and Pearce.[19]
- First Solar example: With the purchase of each First Solar module, funds are set aside to cover the estimated future costs of collection and recycling. These funds pay for all packaging and transportation costs associated with the collection and recycling of the modules. This program follows a three step system: register each module, collect each module once decommissioned and recycle the modules for materials recovery. The one draw back of this example is that it is designed solely for collecting and recycling First Solar products.
- SolarWorld AG example: Company program designed to recycle damaged PV modules of all designs and sizes. Since this program accepts all types of modules, this model may not be economically beneficial for the company and it will be hard to maintain once the need for recycling increases.
- PV Cycle example: Created by the European PV industry as a producer responsible recycling initiative. This program was voluntary until the entry into force of the Recast WEEE Directive with members able to leaving at any time and has become a collective waste management and compliance provider[22] operating under recasted WEEE law in the countries of the European Union and EFTA since.
Current Recycling Process[edit | edit source]
With an expected lifetime of 20 to 30 years, the amount of waste created by PV modules thus far has been relatively minimal; however, as the end of the life of the first PV modules approaches, recycling processes will become increasingly more important. Such recycling processes will need to be environmentally friendly and be suitable for the small amount of modules which are spread over a vast area. It is believed that full-scale recycling of PV modules is still another decade or so away,[23] but it is still important to begin looking at viable recycling options now. A variety of options[23] for recycling PV cells and the companies designing them are explored below:
- Loser Chemie: A universal chemical process which operates at room temperature to reclaim metals from CIS, CIGS and CdTe PV waste. The glass is mechanically separated from the cell, cleaned, and examined for contaminants
- Saperatec: Broken or waste CdTe and CIGS modules are treated with a solution containing a mixture of tensides. This allows the recyclable fractions to be separated from the glass and collected in a filter. The recyclable fraction can then be taken to further treatment and the glass is put in a cleaning tank
- SolarCycle GmbH: A company which is expected to start in 2013 has designed a method which expects to recycle 85% of the input material for crystalline, CIS PV waste and CIGS PV waste. This process entails a coupling of thermal, physical and chemical processing
- First Solar: Recycles thin-film modules such as CIGS. Thus far, the majority of material recycled is manufacturing scrap, along with a small quantity of modules from warranty returns. During the process, PV waste is shredded and crushed; afterward, the semiconductor film is removed via acid and hydrogen peroxide and glass materials are separated from the liquid. The company reports that 90% of glass and 95% of semiconductor can be recovered
Based of the high recovery rate of the First Solar recycling process, the First Solar model is an attractive model for this project. For that reason, the remainder of this project will expand on further analysis and application of the First Solar recycling process. This process was originally based on the recycling of Cadmium Telluride (CdTe) modules, but will now be applied to the recycling of CIGS modules.
In the First Solar recycling process, warranty and end-of-life modules are collected to be shredded with manufacturing scrap. Then, the shredded material is crushed further in the hammer mill. Next the PV film is removed by tumbling the material in a rotating, stainless steel drum. Weak sulfuric acid and hydrogen peroxide are added to the glass to achieve and optimal solid-liquid ratio.[24] At this point, the liquid and solid contents can be separated from one another and from there the process splits. The semiconductor material is precipitated in three steps at increasing pH using sodium hydroxide.[24] Finally, the precipitate is taken to a dewatering stage which separates out the semiconductor material, referred to as the filter cake. This filter cake contains 95% of the semiconductor material and will be processed by a third party. Meanwhile, the laminate is filtered out of the glass-laminate material and the glass in rinsed to produce clean glass cutlet. Through this process, 90% of the glass can be recovered for reuse.[24][25]
Current Viability of Recycling[edit | edit source]
| Determined Value | Units | Gallium | Indium | Glass |
|---|---|---|---|---|
| Area | cm2 | 10000 | 10000 | 10000 |
| Thickness | cm | 0.0004 | 0.0004 | 0.68 |
| Density | g/cm3 | N/A | N/A | 2.6 |
| Mass | g | 6.54 | 10.77 | N/A |
| Percent Recoverable[23] | % | 95 | 95 | 90 |
| Recyclable Mass | g | 6.213 | 10.2315 | 15912 |
Quantifiable Recyclable Materials in CIGS Solar Modules[19]
The calculations of the viability of recycling are based on a 1 m2 solar module. Only the indium, gallium, and glass are considered in the calculations, but the metal or plastic used in the frame and backing can also be recycled. There will be error in these calculations due to differing industrial standards, market values, and assumed inherent properties of the semiconductor.
The profit from recycling the gallium, indium, and glass is calculated using Equation 2.3.1.
Eq. 2.3.1 Profit from Material($⁄module)=mass(g)*value($⁄g)
Equation 2.3.2 determines the mass wasted per m2 module by calculating the usable portion of the solar module and multiplying that by the total mass.
| Determined Value | Units | Indium | Gallium | Glass |
|---|---|---|---|---|
| Mass | g | 6.213 | 10.2315 | 15912 |
| Value[26][27] | $/g | 0.70 | 0.72 | 4.22*10-6 |
| Profit from Material | $/module | 4.35 | 7.37 | 0.07 |
Eq. 2.3.2 Wasted mass(kg⁄module)=(Area(m2)*Power per unit area(W⁄m2)*mass of entire solar module (kg))/(Power output (W))
The value of wasted mass from equation 2.3.2 is then multiplied by the tipping cost, or the price per kg of disposing of solid hazardous waste, as illustrated in Equation 2.3.3.
Eq. 2.3.3 Disposal cost ($⁄module)=Wasted mass (kg⁄module)*Tipping cost ($⁄kg)
| CIGS properties | Value | Units |
|---|---|---|
| Power per unit area[28] | 101.8 | W⁄m2 |
| Mass of entire solar module[28] | 31 | kg |
| Power output[28] | 188.5 | W |
| Wasted mass | 16.74 | kg |
| Tipping cost[29] | 2.76 | $/kg |
| Disposal cost | 46.20 | $ |
| Cost to Recycle | 20.74 | $ |
| Total Profit of Recycling per Module | 37.25 | $ |
Equation 2.3.4 determines the cost of recycling by calculating the product of an experimental cost estimate[20]]] by the power output of a cell.
Eq. 2.3.4 Cost to recycle($⁄module)=0.11 ($/W)* Power output (W)
Equation 2.3.5 determines the total profit by summing the profit of recycling indium, gallium, and glass, and the money saved from not disposing of the hazardous waste. The cost of recycling is subtracted from this value, and it is evident that the recycling process returns a profit, as highlighted in Table 2.3.3.
Eq. 2.3.5 Total profit($⁄module)=Profit from Indium($⁄module)+Profit from Gallium($⁄module)+Profit from Glass($⁄module)+Disposal cost($⁄module)-Recycling cost ($⁄module)
It should be noted here that the example module investigated was a Solyndra module that showed a greater profit from recycling than flat panel modules previously investigated by McDonald and Pearce.
Quantification of Collection Methods[edit | edit source]
Amount Collected[edit | edit source]
| Determined Value | Units | Gallium | Indium | Glass |
|---|---|---|---|---|
| Percent Recoverable[23] | % | 95 | 95 | 90 |
| Recyclable Mass per Module | g | 6.213 | 10.2315 | 15912 |
| Percent Recycled per Module | % | 0.0207 | 0.0341 | 53.04 |
| Total Recyclable Mass | kg | 7.121x103 | 1.173x104 | 1.825x107 |
| Value | $/kg | 700 | 720 | 4.2x10-3 |
| Revenue from Material | $ | 4.98x106 | 8.45x106 | 7.66x104 |
In order to quantify the collection methods, the mass of all the modules in kilograms needs to be determined. Equation 3.1.1 can be used to calculate the total mass:
Eq. 3.1.1 MassTotal(kg) = (TotalPowerOutput(MW)*(1*106W/MW)*MassModule(kg))/PowerOutputModule(W)
Using the values found in Table 2.3.3, the mass of one module is found to be 31kg and the power output for one module is found to be 188.5W. Assuming that 100% of all CIGS PV modules produced can be collected via the variety of collection processes outlined above, a grand total of 209 MW of CIGS PV cells can be collected. Using these values and equation 3.1.1, the total mass of collected CIGS PV will be 3.44x107kg.
Based off of the information found and reported in Table 2.3.3 above, the recyclable mass for gallium, indium, and glass can be calculated. The total recyclable mass was calculated to be 1.827x107kg. Then using the numbers found for the value for each material, the revenue for each material can be calculated. This information was calculated and compiled into Table 3.1.1. These calculations assume a percent recovery of 95% for gallium and indium and of 90% for glass. Note that the remaining 46.91% of the modules will be considered waste as only indium, gallium and glass will be considered in the calculations.
Contaminants and Concentrations[edit | edit source]
Throughout every recycling method there are likely contaminants that could enter into the system. Contaminants such as sulfuric acid, hydrogen peroxide and sodium hydroxide, may enter the system throughout the recycling process. The following reviews how the contaminants enter into the system:
- Sulfuric acid may enter the system as it is added into the process to suspend the solid semiconductor material in liquid
- Hydrogen peroxide may enter the system as it is also added into the process to suspend the solid semiconductor material in liquid
- Sodium hydroxide may enter the system as it is used during the precipitation step of the semiconductor material
With recovery rates of of copper, indium, gallium and selenium at 99.5%, 99.3%, 99.2%, and 99.4%, respectively,[30] the concentration of each containment will be <1% and needs to be further investigated experimentally.
Efficiency[edit | edit source]
After recognizing the percent recovered and the possible contaminants, the percent efficiency of the recycling process can be calculated. The percent efficiency is calculated with use of equation 3.3.1:
Eq. 3.3.1 Efficiency(%) = [TotalRecyled(kg)/(TotalDiscarded(kg)+TotalRecyled(kg))]*100%
Plugging in the values found previously, the efficiency can be calculated as:
Efficiency(%) = [1.827x107kg/3.44x107kg]*100%
Efficiency(%) = 53.1%
Purification Methods[edit | edit source]
CIGS based semiconductor are currently capable of obtaining band gaps ranging from 1.1 to 1.7 eV, along with an approximate efficiency of 19.9%. To obtain such levels in high quality monolith CIGS based semiconductors; elemental copper, gallium, indium and selenium must be 99.99995% pure.[31] The bulk of the purification process relies on a hydrochloric acid and hydrogen peroxide solution, which the materials are placed in. In this step the semiconductor film is removed from the glass. Like other purification processes this step is easily repeatable. Strength of acid solution, duration of soak as well as temperature can easily be modified. This first solution separates out the selenium and allows the copper and indium to separate. Later in the process supported liquid membrane (SLM) and strip dispersion solutions allow the indium and gallium to separate. Further purification of selenium can be obtained by distillation, which involves heating up the selenium (in an inert atmosphere) above its melt temp to remove impure oxides.[30] This second process can be performed if necessary; however, the intent of the process above is to use one acid solution to extract copper, indium, gallium and selenium. Once all the desired metals are in solution, an electrowinning process can remove the pure metals from solution. Therefore we can expect, with a hydrochloric acid and peroxide solution at 10:2, recovery rates of copper, indium, gallium and selenium at 99.5%, 99.3%, 99.2%, and 99.4%, respectively.[30] Product search reveals a potential power plan for the electrowinning process, see below.[32]
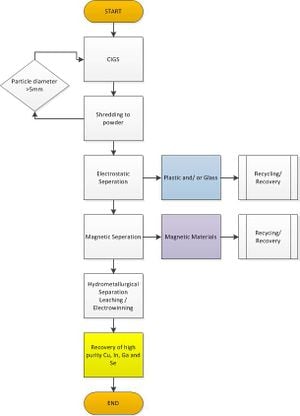
Characterization Methods[edit | edit source]
Numerous characterization tools can be utilized to get an accurate reading of the relative amounts of semiconductor material in a medium. For our particular process, the most obvious candidates are ICP-OES (Inductively coupled plasma optical emission spectroscopy), SEM (Scanning Electron Microscopy) coupled with EDS (Energy Dispersive Spectroscopy) and XRD (X-Ray diffraction).[33]
- In ICP-OES, inductively coupled plasma is used to excite atoms within a material. The electromagnetic radiation emitted from the material produces characteristic wavelengths.Depending on the intensity of the emitted wavelength a elemental concentration can be obtained.
- Using SEM coupled with EDS is an advantageous approach. The SEM allows high magnification imaging to occur as well as allowing to pin point exactly where a chemical analysis may occur. The SEM requires the EDS detector to obtain element information. This analysis technique relies on the development of X-Rays when the electrons in the sample transfer from a high energy shell to a low energy shell.
- XRD relies on the principles of Bragg's equation. With this method a sample is bombarded with X-rays and the intensity of the reflected radiation is recorded. The reflected radiation can be analyzed to determine the reflection angle, &Theta, and to calculate the d-spacing. Both of which can be utilized to develop characteristic peaks of particular elements present in the sample.
Bragg Equation: ηλ=2dSin (Θ)
Alternatives to Production of CIGS PV Cells[edit | edit source]
- The simplest alternative to reproducing CIGS is to sell the extracted materials at market value. This would allow for a smaller facility, lower start up cost, and a greater choice of location, as the facility would not need to be near a glass factory. All the semiconductor and metal materials could be sold for a profit,[26] and the glass has several uses including filtration devices, sand blasting, glassphalt, and more.[34]
- A second alternative is to partner the recycling facility with a current CIGS PV cell producer. The recycling facility would separate the materials of value and ship them next door to the PV cell producer. This would also allow the producer to have the waste from semiconductor deposition refined and recycled, and would ensure a certain amount of stability in the demand for the recycling and refinement facility. Also, when the producer is informed by clients about faulty PV cells, the company could have the cells shipped to the recycling facility.
- Another alternative would be to use the semiconductors in LED lights. Gallium and Indium are used in a broad range of LEDs that create the full spectrum of light. Selenium is also used for a few types, but is not as widely used as gallium.[35]
- A final alternative is using the recycled materials is to produce various other semiconductor devices. Gallium is used in gallium arsenide, a popular semiconductor material, and is needed to make logic chips, lasers, and PV cells.[36] Indium is used in electroluminescent panels, and various other semiconductor materials.[37] Selenium is used in photocopying as a toner, as well as in glass.[38]
Alternative 2 seems like the most optimal alternative to reproducing the PV cells. Transportation costs, collection costs, and initial investment would be kept at a minimum, while profits could be kept at a steady rate. Partnering with a manufacturer creates stability in the business, and a symbiotic relationship would be created. As one company grows, so would the other. Therefore, partnering with a CIGS PV producer is the business development plan that the CIGS recycling facility will follow.
Semiconductor Recycling Facility[edit | edit source]
The location of the CIGS recycling facility is to be located in the south-western United States, as most of the facilities that manufacture CIGS PV cells are located in this region. Also, a large number of PV cells are used in this area, so collection costs can be kept to a minimum.
Recycling Plant Safety Plan[edit | edit source]
As in every work environment where machinery is present, eye protection, ear protection, closed toe shoes, and proper clothing should always be used.
The facility itself will have a large number of hazardous material sensors, rinse stations, fire extinguishers, and emergency exits. The entire facility would be well ventilated, with fume hoods in areas where hazardous fumes and particulates are present. Any and all standards set by OSHA will be followed as well.
The CIGS solar cells to be recycled would need to be fed through a glass pulverizer, which sends fine particles of the solar cell into the air. Due to a small amount of highly toxic cadmium present in the cell, it would be best to crush the glass in an enclosed area, as it is toxic if inhaled and highly corrosive to skin. This would require workers to wear filtration masks as well as Hazmat suits. An alternative option would be to have robotic machines perform tasks in the hazardous environment. They could also handle some of the hazardous chemicals used in the separation and purification of materials. Cost is a major issue for utilizing a robotic operation system, as upfront costs would be much higher than the cost of a recycling process using highly trained individuals. However, insurance costs should be less for a robotic system.
Listed below are the MSDS sheets for the chemicals necessary for recycling CIGS solar cells.[39]
MSDS Sheets
- CIGS Solar Cells
- Di(2-ethyl-hexyl) Phosphoric Acid, D2EHPA
- Hydrochloric Acid
- Sulfuric Acid
- Hydrogen Peroxide
- Sodium Hydroxide
Energy Necessary for Recycling, Collection, and Transportation[edit | edit source]
Recycling and Purification Energy
Equipment Necessary:
- Shredder - Henan Province Sanxing Machinery Co. Shredder (Pat. No: 2L2007200892381.1, 2L200720093087.7, 2L20072093090.9) - 55kW
- ICP-OES - Optima 8000 ICP-OES Spectrometer - 5.3 kW
- Glass Pulverizer - Henan Province Sanxing Machinery Co. Impact Crusher ISO9001:2008 - 55 kW
- Electrowinning Process - Electrowinning Metal Recovery System - 110 kW
- XRD - SAFRAN XRD 3500 - 25 kW
- Total= 270.3 kW
- Work hours in a year= 8765.81 Hrs
- Product processed= 3.44x1010 grams/year
- Total energy consumption of the facility = (8765.81 (Hrs/year)/3.44x1010 (g/year))*270.3 (kW)=6.89x10-5 kW*Hrs/gram
Collection Energy
Due to the lack of CIGS PV cells coming to the end of their life at this time, no data can be found for the mass collection of dead cells. Currently, the only material that needs to be recycled is that found in broken of faulty cells, and scrap from manufacturing.[40] Due to the relatively large size of the PV cells and the number usually installed, it is not viable to rely on drop off stations, so a retrieval system either involving trucks or the shipping industry will probably be employed. An estimated $0.08 per watt is the collection cost,[20] so a true cost depends on the amount of PV cells to be recycled.
Transportation
Ideally, the recycling facility will be located very close, if not next door to a CIGS PV cell manufacturing plant, so transportation costs and energy will be kept at a minimum. This could possibly allow for the use only forklifts. After a recent study by Western Michigan University, it was determined that solar recharge stations and better batteries will provide enough energy for the use of a day's work on a forklift.[41] If this proves to be sustainable, the energy and costs necessary for transportation can be kept at a minimum.
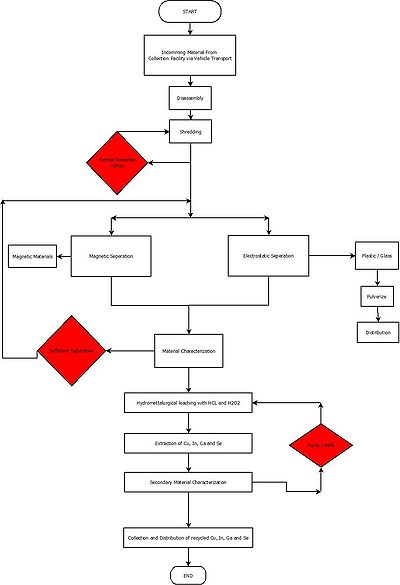
Material Flow Diagram[edit | edit source]
Please refer to the section above for elaboration on the equipment necessary to complete the plant.
Conclusion[edit | edit source]
- This profit value proves the viability of recycling CIGS in photovoltaic cells, so the project now focuses on the potential for post-consumer recycling.
See also[edit | edit source]
References[edit | edit source]
- ↑ "http://www.renewablepowernews.com/archives/2532 (Accessed October 13th, 2012)
- ↑ "CIGS Thin Film Photovoltaic" sponsored by National Centre for Photovoltaic Research and Education: http://web.archive.org/web/20131113111854/http://www.ncpre.iitb.ac.in:80/page.php?pageid=49&pgtitle=CIGS-Thin-Film-Photovoltaic (accessed September 24th, 2012)
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "CIGS Thin Film Photovoltaic" sponsored by National Centre for Photovoltaic Research and Education: http://web.archive.org/web/20131113111854/http://www.ncpre.iitb.ac.in:80/page.php?pageid=49&pgtitle=CIGS-Thin-Film-Photovoltaic (accessed September 24th, 2012)
- ↑ "http://en.wikipedia.org/wiki/Copper indium gallium selenide solar cells (accessed October 14th, 2012)
- ↑ "Revived Interest in CIGS Creating Real Opportunities in Photovoltaics: http://www.nanomarkets.net/Downloads/CIGSPaper.pdf (accessed September 29th, 2012)
- ↑ " http://www.solarserver.com/solar-magazine/solar-news/current/2011/kw48/solibro-reaches-174-aperture-area-efficiency-with-cigs-pv-module.html (accessed October 12, 2012)
- ↑ " http://www.q-cells.com/en/press/article/CIGS-thin-film-technology-reaches-world-record-efficiency-of-174.html(accessed October 12, 2012)
- ↑ " http://en.wikipedia.org/wiki/Miasol%C3%A9(accessed October 14, 2012)
- ↑ " http://en.wikipedia.org/wiki/Copper_indium_gallium_selenide_solar_cells#Sputtering_of_metallic_layers_followed_by_selenization(accessed October 14, 2012)
- ↑ " http://www.sneresearch.com/eng/info/show.php?c_id=4847&pg=5&s_sort=&sub_cat=2&s_type=&s_word=(accessed October 15, 2012)
- ↑ " http://globalsolar.com/company/technology(accessed October 12, 2012)
- ↑ " http://en.wikipedia.org/wiki/Copper_indium_gallium_selenide_solar_cells#Coevaporation(accessed October 12, 2012)
- ↑ " http://en.wikipedia.org/wiki/Global_Solar(accessed October 12, 2012)
- ↑ " http://www.soltecture.com/news/archiv/view/titel/sulfurcell-stellt-erste-cigse-prototypen-vor.html(accessed October 15, 2012)
- ↑ " http://www.pv-tech.org/chip_shots_blog/whats_in_a_name_sulfurcell_is_out_soltecture_is_in_as_german_cigs_thin_film(accessed October 15, 2012)
- ↑ " http://www.nanosolar.com/technology/production-process/(accessed October 15, 2012)
- ↑ " http://en.wikipedia.org/wiki/Nanosolar#Technology(accessed October 15, 2012)
- ↑ "HelioVolt Re-Emerges With Improved Efficiency CIGS Solar Panels: http://www.greentechmedia.com/articles/read/HelioVolt-Re-Emerges-with-Improved-Efficiency-CIGS-Solar-Panels/ (accessed September 29th, 2012)
- ↑ 19.0 19.1 19.2 N. C. McDonald and J. M. Pearce, "Producer Responsibility and Recycling Solar Photovoltaic Modules", Energy Policy 38, pp. 7041–7047(2010). open access
- ↑ 20.0 20.1 20.2 20.3 Fthenakis, V.M. "End-of-life management and recycling of PV modules". Elsevier Ltd. 2000. http://clca.columbia.edu/papers/End_Life_Management_Recycling_Energy_Policy.pdf (Accessed October 14th, 2012)
- ↑ European Commission, "WEEE Directive 2012/19/EU. [1]
- ↑ " National WEEE Regulations come into: http://www.pvcycle.org/press/national-weee-regulations-come-into-force-major-changes-ahead-for-photovoltaic-industry/
- ↑ 23.0 23.1 23.2 23.3 "PV Recycling: The need to be double-green:http://www.solarnovus.com/index.php?option=com_content&view=article&id=3770:pv-recycling-the-need-to-be-double-green-&catid=63:business-features&Itemid=242 (accessed September 26, 2012)
- ↑ 24.0 24.1 24.2 "http://www.renewableenergyfocus.com/view/3005/end-of-life-pv-then-what-recycling-solar-pv-panels/ (accessed October 12, 2012)
- ↑ "http://www.iea-pvps-task12.org/fileadmin/IEA-PVPS_Docs/Documents/Thin%20Film%20-%20CdTe.pdf (accessed October 12, 2012)
- ↑ 26.0 26.1 "Mineral Commodity Summaries 2012:http://minerals.usgs.gov/minerals/pubs/mcs/2012/mcs2012.pdf (accessed October 11, 2012)
- ↑ "Statewide Average Monthly Scrap Value Notice:http://www.calrecycle.ca.gov/BevContainer/ScrapValue/ (accessed October 11, 2012)
- ↑ 28.0 28.1 28.2 "http://www.solardesigntool.com/components/module-panel-solar/Solyndra/SL-001-200/specification-data-sheet.html;jsessionid=FB659F9291306714757547CA740DEAF8 (accessed October 11, 2012)
- ↑ "Statewide Contract FAC53, Category 1, Hazardous Waste Collection and Disposal:http://www.mass.gov/dep/recycle/reduce/fac53c1.pdf (accessed October 11, 2012)
- ↑ 30.0 30.1 30.2 "Method for Recovery of Copper, Indium, Gallium and Selenium Pub. No: US2010/0329970 US
- ↑ "Synthesis of High-Purity Bulk Copper Indium Gallium Selenide Materials Pub. No: US2011/0067997
- ↑ " Allied Plating Supplies http://www.alliedplating.com/store.asp?pid=18509 (Accessed October 13th, 2012)
- ↑ " Recycling Copper Indium Selenide from Solar Cells - Chalmers University of Technology 2009
- ↑ "http://205.153.241.230/P2_Opportunity_Handbook/7_III_4.html (accessed October 11, 2012)
- ↑ "http://en.wikipedia.org/wiki/Light-emitting_diode (accessed October 12, 2012)
- ↑ "http://en.wikipedia.org/wiki/Gallium#Semiconductors (accessed October 12, 2012)
- ↑ "http://en.wikipedia.org/wiki/Indium#Applications (accessed October 12, 2012)
- ↑ "http://en.wikipedia.org/wiki/Selenium#Solar_cells (accessed October 12, 2012)
- ↑ "http://www.google.com/patents/US20100329970 (accessed October 11, 2012)
- ↑ "http://web.archive.org/web/20140221160802/http://earth911.com:80/news/2010/02/19/as-solar-power-advances-disposal-becomes-an-issue/ (accessed October 14, 2012)
- ↑ "http://www.wmich.edu/mfe/mrc/greenmanufacturing/pdf/Borroughs%20Nathan%20Christensen.pdf (accessed October 14, 2012)