VISITECT® CD4
Problem being addressed[edit | edit source]
High rates of HIV infection in the developing world hinders improvements in maternal mortality and also contributes to poor perinatal health outcomes such as stillbirth, preterm birth, and low birth weight. HIV infection of infants and HIV-related maternal deaths are largely due to the delayed initiation of antiretroviral interventions. In addition to mother-to-child transmission of the HIV virus, HIV still remains widespread in low-resource settings.
Detailed description of the solution[edit | edit source]
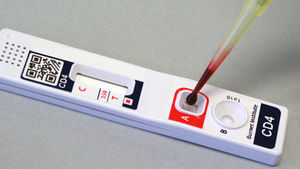
The VISITECT® CD4 enables CD4+ T-cell levels to be determined quickly and conveniently through a finger prick sample. The tests itself only costs US $2, does not require any instrumentation or electricity, and takes forty minutes to yield the results. By assessing CDF count at the first antenatal visit using VISITECT® CD4, pregnant women undergoing the test can receive same day initiation of the most appropriate lifesaving antiretroviral intervention. Administrating antiretroviral intervention early on in the pregnancy not only will benefit the mother's health but will help prevent mother-to-child transmission of the HIV virus, significantly reducing maternal and infant deaths.
Designed by[edit | edit source]
- Designed by:David Anderson from Burnet Institute
- Manufacturer (if different):
- Manufacturer location:Australia
When and where it was tested/implemented[edit | edit source]
Papua New Guinea, Kenya, South Africa
Funding Source[edit | edit source]
Finalist of Saving Lives at Birth Competition
References[edit | edit source]
Peer-reviewed publication[edit | edit source]
Other internally generated reports[edit | edit source]
Parish, T. (July 18, 2012). Project nominated for "Saving Lives at Birth" award. Retrieved from http://www.burnet.edu.au/news/140_project_nominated_for_saving_lives_at_birth_award
Parish, T. (July 26, 2012). Six years in the making: Cd4 test officially launched!. Retrieved from http://www.burnet.edu.au/news/149_six_years_in_the_making_cd4_test_officially_launched
VISITECT® CD4. Retrieved from http://www.omegadiagnostics.com/cd4/
Externally generated reports[edit | edit source]
Performance of an innovative, instrument-free, low-cost, rapid point-of-care cd4 test for accelerating initiation of antiretroviral interventions for HIV 1-infected pregnant women in resource-constrained settings. (August 06, 2012). Retrieved from http://web.archive.org/web/20160507174523/https://savinglivesatbirth.net/summaries/191