Plastics recovery manual/Chapter 2: Plastics
What are plastics ?[edit | edit source]
Although their use has become familiar, plastics are often not well known and confused with each other. They appeared in the mid 19th century, but their true development began in the 60's. Today, their production exceeds that of metals, in volume. They are part of the family of synthetic substances. Their applications are very wide. They range from common objects (bottles, bags,...) to those within the scope of high technology (electronics, aerospace,...). This is due to the multiple properties we can retrieve in these materials.
Generally, plastics are products made from petroleum. We estimate that only 4% of petrol is used in their manufacturing. The world production of plastic is in the order of 150 million tonnes per year. To recycle the plastic waste, it is important to know them well. For this, must start from the basic constituent of plastics: polymers. Mixed with different substances (the additives), these polymers form plastics that can be shaped to obtain marketable plastic objects. After use, these objects constitute a pool of waste plastic that can be recycled.
Polymer + additives = plastics + processing = plastic objects + utilisation = plastic waste

Polymers[edit | edit source]
It is difficult to explain the concept of polymers without discussing the theory behind it. From the chemical standpoint, polymers are composed of "macromolecules"; that is to say, molecules, of very large sizes, that form chains. These chains consist of an assembly of identical links often called "constituent units". These units that are mainly composed of carbon and hydrogen atoms, but we can also find nitrogen, oxygen, chlorine, sulfur,... atoms.
Example Polypropylene (PP) is a commonly used polymer. It is used especially to make milk bottles and to manufacture furniture (chairs, tables,...). The macromolecules that form the PP are of the following type:
Constituent unit
We find that there is a sequence that is repeated all along the chain. It is the "constituent unit". Polymers are often simply represented by their constituent unit placed between brackets.
The n means that the chain consists of a number of n constituent units. This number is variable. It can be very high (up to several hundreds of thousands). This example illustrates the case of a simple polymer. They are not all as this. In fact, the same chain can be composed of different kinds of constituent units and their number in each channel can be variable. In addition, chains can branch, or bind together to form networks.
- Polymer in linear chain
- Polymer in branched chain
- Crosslinked polymer (network)

Also, depending on the different types of constituent units, their number and how they fit together, we get different kinds of polymers. Theoretically, the number of possibilities is almost unlimited. In fact, there are only a few polymers that are relatively simply produced in large quantities. They are called polymers of great diffusion. The others, the "engineering polymers" are used in more specific applications and often in high technology. They are generally more expensive!
Generally, the polymers are immiscible, that is to say we can not mix them together to obtain a homogeneous material. This finding has a serious consequence: It is necessary to sort the plastics before recycling
We must therefore identify the base polymer from which all plastic waste is manufactured. This identification can not be done without some knowledge of the major polymers. These fall into two broad families: thermoplastics and thermosetting polymers.
a. The thermoplastic polymers, "Melt when we heat them"
The thermoplastic polymers soften when increasing the temperature. This is due to their linear or branched structure. When heated, the chains separate and slide against each other: they become malleable. These thermoplastic polymers can thus be remelted and molded without their properties are modified (too) much.
b. The thermosetting polymers, "Remain hard when we heat them"
Once molded, thermosetting polymers take a final form. Their structure is crosslinked. The chains are thus interlinked and can not move. The thermosetting polymers can not be remelted. We can not recycle them mechanically.
Notes:
- Whether thermoplastic or thermosetting polymer, polymers are combustible materials: they burn well. Subjected to high temperatures, the chains eventually break and the polymer is degraded irreversibly.
- Contrary to some misconceptions, rubbers are not plastic materials but elastic materials; "they resume their original shape after being expanded! They are also called elastomers. They are
mainly used in the manufacturing of tires, shoe soles, rubber bands, tubes, belts,...
Thermoplastics are by far the most widely used polymers. There are several hundreds of types, but fortunately, only fifteen of them are used for common applications. Four thermoplastics have a share over 70% of all the polymers in the global market: polyethylene, polypropylene, polystyrene and polyvinylchloride (commonly known as PVC). Their ease of transformtaion and the large quantities that are produced make that they are good candidates for recycling. This guide will therefore be limited to the recycling of these four polymers and will discuss them in detail. For simplicity, the thermoplastic polymers are simply called "plastics" in the remainder of this book.
Polyethylene: PE Polyethylene (PE) is the most common polymer. It is also the one with the simplest structure. The chains are only composed of carbon atoms, each linked to two hydrogen atoms.
Constituent unit
It may be represented simply by its constituent unit placed between brackets.
Depending on the structure of their chains, the polyethylene is separated into two categories:
High density polyethylene (HDPE) and low density polyethylene (LDPE).
Unlike other polymers, HDPE and LDPE are miscible. They can be recycled together. However, to maintain their specific properties, it is better to separate them.
LDPE has a branched structure. It is flexible and soft to the touch. If it does not contain any pigments, it is transparent when very thin (film) and milky white when thick. It is used to make agricultural and packaging film, toys, hoses, bags, tubes, bottles, screw or snapped corks,...
HDPE instead has a linear structure. It is stronger, both in mechanical terms (hardness, stiffness,...), as well as thermal terms. It is opaque regardless of thickness. It is used in the manufacturing of industrial packaging films, bottles, milk bottles, jerry cans, buckets, caps, toys, fuel tanks, reusable racks,...
Polypropylene: PP Just as polyethylene, polypropylene (PP) is a polymer which is only composed of carbon and hydrogen atoms. Its structure consists of linear chains (see Figure 2.2 and 2.3). This is one of the most versatile polymers. Like EP, it is opaque if it does not contain any pigment. It is however more rigid, is more resistant to heat but it is more susceptible to cold. It is used especially in the manufacture of yoghurt and margarine pots, the different reservoirs for car fluids (except fuels), the parts of electrical appliances, boxes of car batteries, furniture (chairs, tables,...), bottles, etc.. The impact resistance of PP is greatly appreciated, among others for making bumpers for cars. Another great application is its use as textile fibers.
Polyvinyl chloride: PVC Polyvinyl chloride is better known as PVC. This is one of the oldest polymers (1931). The constituent unit of the chains is composed of carbon and hydrogen atoms, but also contains a chlorine atom.
PVC is a hard and rigid polymer. It can be transparent. It is used mainly in the manufacturing of pipes (pipes, gutters,...) and profiles (window frames,...). To make it more flexible, we add 10 to 50% "plasticizers". These complex molecules are intercalated between the polymer chains so as to reduce its rigidity. It is then called "PVC plastic". It can be used to make inflatable objects, shoes, boots, balls, hoses for water and gas, for the insulation of electric cables,... PVC and "PVC plastic" can be recycled together. Properties (stiffness) of the resulting product will depend on the proportions in which they are combined.
Notes: Although the constituent unit of PVC contains one atom of chlorine, this component represents 57% by weight of the polymer. When burning, the chlorine atom is released and inhibits the combustion. In other words, a PVC object on fire will tend to disappear. This gives PVC a very good fire resistance. However, it releases hydrochloric acid (HCl) which is toxic and corrosive.
Polystyrene: PS The structure of polystyrene (PS) may seem more complex than those of previous polymers. Similar to PP and PVC, a hydrogen atom is substituted by a "benzene ring", that is to say a ring composed of carbon and hydrogen.
This ring is traditionally shown schematically by a circle inscribed in a hexagon. The constituent unit of polystyrene is then outlined as follows:
PS is popular in food packaging for its excellent chemical stability. Several kinds of PS exist of which three are common:
- standard polystyrene (PS): It is hard, rigid and transparent but brittle and not very resistant to shock. It is used in appliances, disposable cups, audio and video, clothespins,...
- High impact polystyrene (SB): By changing somewhat its structure, the PS is made more flexible to better resist shock. It is then used for the packaging of dairy products (yoghurt, margarine, toys, boxes of small appliances,...)
- expanded polystyrene (EPS):
When we add a foaming agent, the PS expands very well. It is possible to obtain a product containing 96 to 98% air. This property of PS is widely used in food packaging, but also for insulation (frigolite).
Other polymers The four polymers described above represent a large proportion of polymer products (over 70%). In these polymers of great diffusion, we must add the polyethylene terephthalate (PET). This thermoplastic polymer is increasingly replacing PVC for the manufacturing of bottles.
| Polymers | Abbreviation | Objects |
| Polyacrylonitrile/Butadiene Styrene | ABS | dashboards, electric appliances,... |
| Polymethyl methacrylate | PMMA | car lights, Plexiglas... |
| Polytetrafluoroethylene | PTFE | antifriction parts (better known under the name Teflon) |
| Polyamides | PA | Fiber textiles |
| Polycarbonate | PC | Compact discs |
| Polyurethane | PUR | mattress foams, chairs,... |
| Epoxides | EP | adhesives, resins, varnishes,... |
| Polyesters | UP | corrugated plates... |
| Phenoplastes |   | adhesives, varnishes, foams,... |
| Aminoplastes |   | binders, dishes,... |
Table 2.1: Other polymers Note: lighter blue (acqua)= thermoplastic polymer; darker blue (acquamarine)= thermosetting polymer
Nevertheless, we must not forget that there are many other polymers. As a reference, Table 2.1 gives a partial list of them as well as some of their current applications. The recycling of these polymers is not discussed in this book for two reasons:
- Or the limited quantities available does not justify recycling;
- Or recycling requires the use of tools which are too expensive and/or sophisticated.
To learn more about polymers:
General course of organic chemistry (not only on polymers)
Search: "plastics" Summary, in 6 pages, of the main polymers.
Specific course on polymers (5 levels of difficulty)
Description of different polymers (chemical structure to the formatting)
- http://chemphys.u-strasbg.fr/ ~ mpb / teach / HDPE /
Site dedicated to polyethylenes
Site for all information on PVC (general, safety, recycling,...)
Union of Producers of plastics. Website sells plastics, providing lots of data on their specifics.
Plastics[edit | edit source]
The polymers are manufactured in large industrial reactors. They could be used as such, but they usually add various additives to receive better properties. The mix "polymers + additives" is called "compound premix" or simply "plastics". It is not important to know in detail what these agents are, but it is important to know whether they are present in different proportions in the polymers.
- Antioxidants and stabilizers
Antioxidants reduce the degradation of plastics by oxygen and by airborne pollutants. They are found mainly in the PE and PP. Stabilizers limit the degradation of plastics from heat and light, particularly for PVC which is very susceptible to these attacks.
- Plasticizers
Plasticizers are substances that are added to soften rigid polymers. They are mainly used for PVC (plasticized PVC see above).
- Foaming agents
These agents are used to transform certain polymers into foam. In general, this hydrocarbon has a low boiling point. The best known case is that of expanded polystyrene (frigolite).
- Flame retardants
Flame retardants are added to reduce the flammability of plastics. They are present in large quantities, including all plastics used for electrical applications (small appliances, cables, switches,...).
- Pigments and dyes
Dyes and pigments are substances that are added in small quantities (0.1 to 5%) to give a color to plastics. Dyes are organic liquids that give plastiques color by dissolving into polymers. The thus obtained plastics can retain their transparency or their translucency. Pigments color polymers by scattering between the polymer chains. The pigments used are often oxides or salts of metals: titanium dioxide (white), iron oxide (red), cadmium sulfide (yellow), black carbon (black),... The obtained plastiques are always opaque.
- Filler and reinforcements
Fillers are cheap materials, incorporated into plastics to reduce their costs by 5-60%, but also to improve their physical, mechanical and thermal properties. These changes are more or less important depending on the nature (mineral, vegetable, synthetic,...) and form (powder, fiber,...) of the filler.
- Lubricants
These additives are incorporated into polymers to provide internal and external lubrication. The external lubrication reduces friction between the polymer and the metal walls of the equipment that needs repairing. The internal lubrication promotes the flow of polymers and limits damage due to shear in the machines. Rigid PVC requires internal lubricants; without it it will deteriorate during its first use. The PE, PP and PS should also be lubricated. Many lubricants perform both functions (internal and external lubrication).
The proportions of the various components of a plastic (polymer + additives) constitute its "formulation". When buying plastics, they are usually in the form of pellets already containing the different additives according to the formulations proposed by the producers. Only the pigments are usually added by processors.

The plastic objects[edit | edit source]
The conversion of plastic pellets to marketable objects is made possible trough the use of processing machines. The principal methods of shaping are described in Chapter 5. At this stage it is important to make two remarks:
- A plastic object can be composed of different kinds of plastics;
- A plastic object can contain other materials than plastic.
For example, consider the simple case of an oil can. Apparently, it is a beautiful HDPE object which is interesting to recycle. When examined it in detail, we find that it is equipped with:
- A cap and a locking ring;
- A label.
The cap and the ring often have a different color than the can and can be made from another polymer. The paper label or plastic is glued to the bottle.
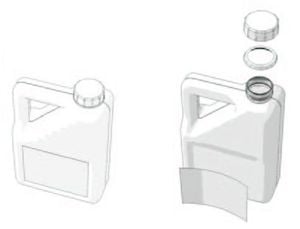
These components may represent 5 to 10% of the weight of the can. They will disrupt the recycling process. We must therefore separate them. The cap and ring can be recycled separately. The label and the glue should be properly discarded from the can.
This example illustrates the concept of Level of Contamination (DC). This is the ratio (expressed in percent) between the weight of the materials other than the plastic material which we desire to recycle and the total weight of the object; that is to say:
In the case of the oil can, if you want to recycle only the plastic container, the contaminating materials are the cap, the ring, the paper and the glue. We find:
This means that for a batch of 100 kg cans, 5 to 10 kg is not recyclable. The degree of contamination is zero for objects made solely from a single plastic (beer trays, chairs, basins,...). It can sometimes be very high. A glaring example is that of car batteries. The tray (made of PP) weighs practically nothing compared to the rest (lead, acid, metal frames,...). The degree of contamination can reach 90%.

Note: It is important to introduce the concept of "multilayered packages". As the name indicates, these packages consist of a series of layers of different materials: paper, cardboard, aluminum, plastics,... They are closely attached together. It is rarely possible to separate them for recycling. A familiar example is the milk carton. It is actually composed of several layers of cardboard, aluminum and PE. It is not possible to separate the different layers to recycle the different materials on an isolated manner.
Plastic waste[edit | edit source]
After use, the objects become waste plastics which users want to get rid of. Some plastics, such as packaging, become waste quickly; others take several years: movable parts for cars or appliances, plastics used in construction... During their lifetime, waste plastics are more or less altered and fouled. The alteration is the modification of the chemical structure of the polymers. It is a result of the natural aging of the polymers but also due to oxidation, heat, light... The fouling may have two origins:
- Fouling from own waste: these come from residues that were still contained within the waste. It mostly concerns containers and packaging, for example leftover shampoo in a bottle, residue left over in a pot of margarine,...
- Fouling from external sources: this is filth accumulated by the waste material before being recovered. This filth may be of a certain quantity if the waste was mixed with other waste or if it had lain on the ground.
The degree of filth (DF), is the name given to the percentage of dirt the waste carries. They are unique to the waste or external source. It is determined by weighing the waste before and after the washing:
The degree of fouling of the waste is an important parameter when evaluating a recycling project. The higher it is:
- the longer and difficult the washing of the waste is;
- the less amount of the plastic is left over.
Also, the waste coming from landfills has a degree of fouling that may reach up to 25%. This means that for every 100 kg of recovered plastics, there is also 25 kg of filth. Only 75 kg can be sorted.

Waste plastics are however far less dirty when collected from homes. The degree of pollution is then less than 10%.

How to identify plastics ?[edit | edit source]
The identification of plastics is a crucial step in the recycling process. Indeed, as explained above, the plastics mix poorly when we melt them for the reshaping. The step of sorting is thus always necessary. Everyone knows the difference between a copper wire and steel cable. However, the recognition of the different kinds of plastics is less obvious and bear resemblences. Moreover, within the same family, they can have very different appearances. Identification requires a certain degree of practice, but it quickly becomes a breeze. It is estimated that it takes two weeks for a person to become familiar with the recognition and sorting of the main plastics. When in doubt, only testing can allow someone to determine whether a plastic belongs to one or the other category. These tests are described in the following paragraphs in order of difficulty of implementation. When plastic could not be identified with certainty, we must not forget that it may be part of another family than PE, PP, PS or PVC.
General criteria[edit | edit source]
Some general properties of plastics are included in the following table. They can serve as a starting point for recognition.
| Type | Flexibility | Perfect transparency | Miscellaneous |
| HDPE | Flexible | no | Mottled films |
| LDPE | Very flexible | no,except for films | Touch like wax, films difficult to tear |
| PP | Stiff, but does not break | no | |
| PVC | Breaks unless plasticized | yes | Rarely used, transparent except for bottles |
| PS | Rigid, brittle | yes |   |
| PET | Very flexible | yes |   |
Table 2.2: General properties of plastics
It is difficult to compare the plastics according to their flexibility. This varies according to their thickness. The criterion of transparency is clearly valid only if the plastics were not fouled. Another interesting property of the plastics is their "penetrating power"; this can be estimated using the punch test.
Punch test: This test is done using a piercing tool (e.g. perforator, punch belt, pointer,...). When one wants to perforate a piece of PE, PP or PVC plastic, it is necessary to exert a continuous pressure, so we feel the piercing tool entering the plastic. However, PS, PET and rigid PVC are punctured with a dry stroke accompanied with a breaking sound.
Marking systems[edit | edit source]
For several years, some countries (USA, Canada, Japan, Australia, Europe,...) require that the plastics on the market are marked by the international numbering system:

| English | PET | HDPE | V | LDPE | PP | PS | Others |
| French | PET | PEHD | PVC | PEBD | PP | PS | Other |
Table 2.3: International marking system
This greatly facilitates sorting. These acronyms are either placed in relief on the back of molded parts, printed on extruded parts or on films:
Shaping marks[edit | edit source]
Plastic objects retain marks from their shaping. These "scars" are different depending on the transformation technique used. These techniques described in chapter 5 are more or less applicable depending on the type of plastic. From the shaping marks, one may determine which technique was used and thus get an idea of the type of plastic. The following table gives the most common transformation methods and their importance in light of the major plastics.
| HDPE | PVC | LDPE | PP | PET | |
| Injection | * | * | * | ** | * |
| Extrusion | ** | *** | * | ** | * |
| Extrusion-blowing | ** | * | little | * | no |
| Injection-blowing | ** | * | little | * | *** |
| Film | * | *** | *** | * | * |
Table 2.4: Transformation of plastics
We note that almost all methods are used for all types of plastics. The classification of waste plastics on the basis of their shaping marks has thus limited applications. It is nonetheless a very rapid method in specific cases.
Example: An important example is the distinction between the PVC bottles and PET bottles. PVC bottles are made by extrusion followed by the blowing in a mold. The base of the bottle has a linear scar that is a consequence of the closing of the mold. PET bottles are however made by injection-blowing. The base of the bottle possesses in this case a circular injection mark.
There are less and less PVC bottles to be found. These are gradually being replaced by PET bottles, which are more impervious to gas and more resistant to pressure (soft drinks).


Note:
It is often helpful to separate the plastic objects according to their methods of transformation. Indeed, as explained above, the plastics are composed of polymers in which additives are added, including lubricants and plasticizers. These are introduced in varying proportions depending on the applications. On the plastics market, we find eg "HDPE for injection" or "HDPE for films". It's the same polymer but the producers have added additives facilitating respectively injection or filming. Although they can be recycled together, we prefer to separate them to get recycled plastic with the same properties.
Fingernail scratching (hardness test)[edit | edit source]
The hardness test consists of trying to scratch the plastic with the fingernail. This easy test is not always reliable. It can give an initial idea but often requires confirmation. The following table gives the different outcomes of plastics being scratched by a fingernail.
| Type | Fingernail scratch |
| HDPE | Easily |
| PVC | Rigid = no; plasticized = yes |
| LDPE | Yes, easily |
| PP | No |
| PS | No |
| PET | No, or very little |
Table 2.5: Hardness of plastic
Flame test[edit | edit source]
The flame test is fast and reliable. It involves burning a piece of plastic and observing its behavior: flammability, color flame, smoke, odor, droplets,...
Test Description: Cut a strip of plastic ± 5 cm long and ± 1 cm wide. Bring it above the flame of a lighter, above an inert surface (eg an ashtray).
Interpretation: By observing the behavior of the plastic in contact with the flame, it is possible to identify from the following criteria:
- Ease of combustion
- Color of the flame
- Droplet formation (inflamed or not)
- Color of the smoke
- Odor from burning
These different criteria of classification are shown in the following table for the major plastics:
| Type | Combustion | Flame | Droplets | Smoke | Odor | Miscellaneous |
| HDPE | easy | blue base, yellow top | many inflamed | white | candle | |
| LDPE | easy | blue and yellow | many inflamed | white | candle | |
| PVC | the flame extinguishes | yellow edges, green lower extremeties | black | black | spicy (chlorine) | coal residues |
| PP | easy | yellow flame, blue base | many inflamed | white | candle, but less strong | |
| PS | easy | yellow-orange | inflamed | black with flakes | gas, sweet | blisters, black fumes |
| PET | difficult to inflame | yellow | yes, but few in number | slightly black | weak buttery smell |
Table 2.6: Flame test
Recommendations:
- Perform the test in a well ventilated space, avoid inhaling fumes;
- Do not use candles, the black smoke of these can give way to errors;
- Perform the test over a suitable surface (eg ashtray, stone plate, metal,...) to avoid any risk of fire due to burning and/or inflamed droplets.
It is important to remember that some plastics contain flame retardants. They can change the behavior of inflamed plastics.
Density test[edit | edit source]
The density test is based on the following principle: "A body immersed in a liquid floats or sinks depending on whether its density is lower or greater than that of the liquid" It is therefore possible to obtain information on the density of a piece of plastic when the plastic is immersed in a liquid with a known density:
- If it sinks, its density is greater than that of the liquid;
- If it floats to the surface, its density is lower than that of the liquid.
The densities of major plastics are between 0.90 and 1.40 kg/dm³:
| Type | Density (kg/dm³) |
| PP | 0.90 |
| LDPE | 0.91 to 0.93 |
| HDPE | 0.94 to 0.96 |
| PS | from 1.04 to 1.10 |
| PVC | 1.30 to 1.35 |
| PET | 1.40 |
Table 2.7: Density of the major plastics
By using liquids of different densities, it is possible to classify the plastics successively according to their category. Although the principle is simple, the density test is more time consuming to implement than the others because it requires multiple manipulations. It is very reliable unless the plastics are heavily filled. Fillers (and other additives) change the density of the plastics.
Material:
- Containers of one liter minimum, preferably transparent;
- A cutting tool to remove plastic chips: cutter, perforator,...
- Cooking salt (NaCl);
- Ethanol, methanol: up to 110 ml per liter. Buy a product for which the density is accurately indicated on the bottle;
- A tool to mix the solutions.
Test Description:
Take a small piece of plastic the size of a confetti with a cutter, a perforator, a punch for belts,... and then plunge it into a liquid of a known density.
Different liquids can be used to test density. The simplest liquid is water (γwater= 1 kg/dm³) with which we can differentiate PP, LDPE and PEHB of PS, PET and PVC. With their density below 1, PP, LDPE and PEHB float while the others sink. To differentiate PS and PVC, we can use water saturated with salt (NaCl). Such a solution is obtained by adding salt in water. The solution is saturated when it is no longer possible to dissolve more salt. We can see salt crystals on the bottom. The density of the liquid is then more or less than 1.2 kg/dm³.
In such a liquid, PS floats while PVC and PET sink.
The differences between PP/HDPE and LDPE/PEHB are more difficult to determine, but can be done with a liquid of a density below 1 kg/dm³; which is obtained by mixing water and alcohol. To know the quantity "x"(in ml) of an alcohol density of ?alc. to be added to one liter of water to obtain a liquid with a density of γsol., we can use the following formula:
The alcohols that are easiest to find in shops are methanol (density = 0.79 kg/dm³) and ethanol (density = 0.81 kg/dm³)[1] A liquid of a density equal to 0.93 kg/dm³ can be obtained by adding 500 ml of methanol or 580 ml of ethanol in a liter of water. In such a liquid, HDPE sinks while the PP and LDPE float. For liquids with other densities, we use the formula above, or the following table. It gives the amount of alcohol added to one liter of water for a liquid having the desired density.
| Density (kg/m³) | Methanol (ml) | Ethanol (ml) |
| 0.90 | 920 | 1100 |
| 0.91 | 760 | 890 |
| 0.92 | 620 | 720 |
| 0.93 | 500 | 580 |
| 0.94 | 400 | 460 |
| 0.95 | 310 | 350 |
| 0.96 | 240 | 260 |
| 0.97 | 170 | 190 |
| 0.98 | 110 | 120 |
| 0.99 | 50 | 60 |
Table 2.8: Density of mixtures of water + alcohol
In practice, it is difficult to separate PP and LDPE using this test. Their densities are too close. The fingernail scratching test however allows to differentiate them.
Precautions:
- Alcohols are highly inflammable and relatively toxic! We must prepare the mixtures in a well ventilated area and avoid inhaling vapors. We must also put these products out of the reach of children.
- Alcohol evaporates quickly. After completing the test, it is necessary to close the container.
- Impurities (fine particles and residual liquid) may change the density of the solutions. It is necessary to replace them once they begin to become cloudy in appearance.
The density of liquids can be controlled using a hydrometer. This instrument consists of a floating glass, weighted done with lead and mounted over a graduated rod.
The float sinks in a liquid until the weight of the displaced liquid corresponds to the weight of the float (Archimedes principle). The hydrometer floats higher or lower depending on the density of the liquids. Graduations are calibrated to correspond with the surface liquid.

Hydrometers cost around 25 to 75 €/piece excluding postage costs. An accuracy of 1-2g/l is recommended. We must ensure that the density range covered is between 0.850 and 1.250 kg/l. For this, it is often necessary to purchase two.
We can also make our own hydrometers, but it is often difficult to obtain the level of precision required for the separation of the plastics.
Websites to obtain a hydrometer:
- www.labomoderne.com
- www.vwrsp.com
- www.bioblock.be
- www.coleparmer.com
Specific tests for confirmation[edit | edit source]
In addition to the general tests described above, there are also other more specific tests to differentiate certain types of plastic.
- PVC: melt a small amount of plastic by pressing a copper wire in a sample. Heat this thread on the flame of a lighter. A green flame indicates the presence of chlorine in the PVC.
- PVC-PET: plunging them into hot water. PVC softens, but PET doesn't.
- Bags are generally made of HDPE and LDPE. HDPE bags are more mottled and always opaque.
- Thermoplastics-thermosets: drive a heated wire in a sample. If it penetrates, is a thermoplastic, otherwise is a thermoset.
- Rubber: just apply pressure with the fingernail. Rubber will deform but rapidly retake the original shape.
Note:
Identifying plastics quickly becomes a habit, and tests are used more as confirmation tools. It is interesting to build up a database with the main types of frequently used plastic objects. This can be done with a copy of each shown on top of a shelf. Each object needs to be labeled.
References[edit | edit source]
- ↑ propanol (density = 0.78 kg / dm ³) can also be used if others are too difficult to find.
