Open source ventilator
Over the rest of the semester MOST and well as the MTU Open Source Hardware Enterprise will be documenting, building and testing open source ventilators. If you want to help please do.
- Pearce JM. A review of open source ventilators for COVID-19 and future pandemics. F1000Research 2020, 9:218 (https://doi.org/10.12688/f1000research.22942.1) academia open access
- 2020 Michigan Tech Open Sustainability Technology Group COVID19 Projects & Publications
Background[edit | edit source]
Y-Hacker News,"Open-Source "pandemic ventilator", 2020.
- Drugs and expertise are required along with the use of a ventilator.
- Clincal and professional care is required to use a ventilator safely. It is a risk to use a homemade ventilator at home due to clogging of fluids.
Dreamer, "Pandemic Ventilator Project", 2020 March 28.
- Must be able to manually control the peak inspiratory pressure (PIP), tidal volume, inspiration to expiration ratio (i:e), breathes per minute (BPM), and peak end-expiratory pressure (PEEP).

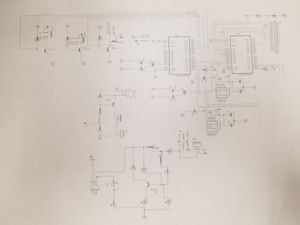
The schematic for this open-source ventilator
Reddit, "Open Source Crowd Sourced Medical Ventilator", 2020.
- Possible short term solution would be connecting multiple patients to a single ventilator that would allow for adjustments of the PIP value. This is complicated when considering pandemics such as COVID-19.
- First single-use ventilator: https://mms.mckesson.com/product/1115066/Hartwell-Medical-SV2131B (not advised for use).
Gerrit Coetzee, "ULTIMATE MEDICAL HACKATHON: HOW FAST CAN WE DESIGN AND DEPLOY AN OPEN SOURCE VENTILATOR?", 2020 March 12.
- The problem with creating an open source ventilator is the decision of what kind of respirator is needed (CPAP, BIPAP, Hi-Flo oxygen NIV).
- A Nasal cannula-based NIV would be needed for ventilators specific to COVID-19.
Coronavirus Tech Hanbook, "Other Hardware", 2020.
- Document that has numerous references for COVID-19.
Gareth Branwyn, "FB group forms to open-source development of coronavirus-related medical hardware", 2020 April 4.
- 15-20% of patients who get COVID-19 will require hospitalization.
- Rice Engineering open source ventilator: https://www.youtube.com/watch?v=1t2t8d8xtD0&feature=youtu.be
- 3D-printable door opening device to decrease the spread of COVID-19 through door handles: https://www.thingiverse.com/thing:4192643?fbclid=IwAR2Fwcq6l-oymUiYwW0H44xhcTO1EFPHY2BfitPrqdL-5NVCN0S8Jof7BKY
"Open-Source COVID19 Medical Supplies Requirements Open-Source COVID19 Medical Supplies Requirements", 2020.
- Document that describes the medical devices that may become short supply during the COVID-19 pandemic.
- Non-invasive ventilation machines (CPAP, BIPAP, hi-flo oxygen) are discouraged since they aerosolize the virus during exhalation.
- The real problem may be the short-staffed respiratory therapists, and the amount of time to train them on invasive respiratory methods, and the capacity of hospitals than the possibility of running out of ventilators.
Davide Sher, "Italian hospital saves Covid-19 patients lives by 3D printing valves for reanimation devices", 2020 March 14.
- 3D-printed valves for ventilators work, however they are patented so the STL files are not given. Only hospitals can 3D print the part during an emergency
- Venturi valves are being used: https://www.sciencedirect.com/topics/medicine-and-dentistry/venturi-mask
- STL file of the venturi valves are not given due to the hospital requirement being fulfilled. Only in an emergency can 3D printing produce a replica of medical components, and it requires expansive machinery.
"Forum AIR".
- List of articles and announcements pertaining to the importance of COVID-19.
Lea Simpson, "Frontier Tech 4 COVID Action: emerging market ventilation systems", 2020 March 16.
- The UK is looking for reliable rapidly manufactured ventilation systems.
- The RMVS must:
* Be reliable (work for >= 14 days at 24 hours/day). * Provide two settings for volume of air/air O2 mix deliverd per cylce/breath (450 ml +/- 10 ml per breath and 350 ml +/- 10 ml per breath) * Provide the air/air O2 mix at a peak pressure of 250 mm H2O * Patient supply pipework to remain pressurized at all times to 150 mm H2O * Have an adjustable rate of 12-20 cycles/breaths per minute. * Deliver at least 400 ml of air/air O2 mix in no more than 1.5 seconds. * Built from O2 safe components. * Capable of breathing for an unconscious patient. Ability to sense when a patient is breathing is desirable. * Supply pure air and air O2 mix at a range of concentrations (at 50% and 100% O2). * Support connections for hospital O2 supplies. * Be compatible with standard COTS catheter mount fittings (Male: 15 mm; Female: 22 mm). * Fail SAFE: having alarms for at least pressure loss and O2 loss.
MHRA, "Rapidly Manufactured Ventilator System (RMVS)".
- Manual about the UK requirements that a RMVS must have in order to be used during an emergency.
- Refer to pages 5-6 for ventilation requirements.
- Refer to page 7 for infection control.
- Refer to pages 8-9 for the required alarms and biological safety.
- Refer to pages 10-11 and Appendix B for testing requirements/protocol.
Montreal General Hospital Foundation, "Code Life Ventilator Challenge".
- The objective and rules for a ventilator challenge in Montreal.
How Many are Needed?[edit | edit source]
See Dave Denkenbergers' spreadsheet
- https://www.youtube.com/watch?v=jmUMolg-7nA
- https://www.nytimes.com/2020/03/13/us/coronavirus-deaths-estimate.html
- https://www.politico.com/news/2020/03/14/health-system-coronavirus-preparation-129066
- https://www.npr.org/sections/health-shots/2020/03/14/815675678/as-the-pandemic-spreads-will-there-be-enough-ventilators
- https://abcnews.go.com/Health/demand-ventilators-spikes-coronavirus-looms/story?id=69597233
- https://www.nytimes.com/2020/03/12/opinion/coronavirus-hospital-shortage.html
- FDA sweeping away regulations https://www.precisionvaccinations.com/continuous-positive-airway-pressure-cpap-machines-enhance-people-respiratory-challenges ,
Existing Designs[edit | edit source]
- https://github.com/PubInv/covid19-vent-list -- the list now updated daily
https://simulation.health.ufl.edu/technology-development/open-source-ventilator-project/ Florida GPL and nearly there
- https://www.instructables.com/id/The-Pandemic-Ventilator/
- https://www.appropedia.org/Negative_pressure_respirator
- https://app.jogl.io/project/121#about and https://www.projectopenair.org/ and the core documentation on the ventilator project https://drive.google.com/drive/folders/1TrHL40-RwSL_yWivaRF8GyKmLVJMr63j
- https://www.medonegroup.com/pdf/manuals/techManuals/Cardinal-Health-VELA-Ventilator-Service-Manual.pdf Service Manual for a Commercial Ventilator
- https://www.cbc.ca/news/canada/london/pandemic-ventilator-coronvirus-hospitals-1.5493830
- https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2044.2009.06207.x
- https://www.oxygen.protofy.xyz/
- https://e-vent.mit.edu/
- https://gitlab.com/open-source-ventilator/OpenLung
- https://simulation.health.ufl.edu/technology-development/open-source-ventilator-project/
- https://opencovidpledge.org/
- https://www.medrxiv.org/content/10.1101/2020.03.24.20042234v1.full.pdf
- https://gitlab.com/reespirator
- https://op-vent.stanford.edu/
- http://mvm.care/
- A brief for engineers, by a doctor, on hacking a ventilator for surge capacity in Covid19 patients.
MTU Designs[edit | edit source]
Open Source Ventilator[edit | edit source]
- Details currently in progress
Innovative Global Solutions (IGS) Enterprise[edit | edit source]
IGS enterprise has given its focus to constructing three versions of a pandemic ventilator throughout the past few years. These specifications were given for use of either rebuilding the machine to be used during the COVID-19 outbreak or taking key components to use in the open source ventilator (see above). This ventilator had achieved a price tag of below 2000 USD, the most expensive component being the compressor.
Testing Procedures[edit | edit source]
- https://www.gov.uk/government/publications/specification-for-ventilators-to-be-used-in-uk-hospitals-during-the-coronavirus-covid-19-outbreak
- https://github.com/PubInv/ventmon-ventilator-inline-test-monitor
- ISO 80601-2-12 https://www.iso.org/standard/72069.html
- MDSR Minimum Requirements: http://www.mdsr.ecri.org/summary/detail.aspx?doc_id=8305
MTU Testing Procedure[edit | edit source]
- Select on of the four modes using the user interface on the ventilator (for the IGS ventilator):
*Continuous mechanical ventilation (CMV): used when a patient is not breathing on their own *Inverse ratio ventilation (IRV): used when a patients inhale lasts longer than their exhales(i.e 2:1 instead of 1:2) *Pressure support ventilation (PSV): when a patient has trouble completing a breath thus resulting in negative pressure during a breathing circuit *Assist Control (AC): used when a patient needs support for every breath
- Confirm that the peak inspiratory pressure (PIP), respiratory rate (RR), positive end expiratory pressure (PEEP), inspiratroy:expiratory ration (i:e), and tidal volume (TV) are accurate by using the Michigan Instruments software package that accompanies the lung (downloaded in the MI USB drive).
- Test the alarms by unplugging the machine, wiring, and appropriate tubing. The accessories that should be tested are:
*Low Pressure Alarm: Decrease the PIP value using the ventilators user interface to an abnormal value. *High Pressure Alarm: Increase the PIP value using the ventilators user interface to an abnormal value. *Oxygen Concentration Alarm: Increase/decrease the percentage of oxygen per breathe to above/below the safety range (19.5-23.5%) by mechanically increasing/decreasing the amount of O2 entering the ventilator and using an O2 sensor. *Battery Backup Alarm: Unplug the ventilator's primary battery from the voltage source. *Wire Disconnection Alarm: Unplug a wire from the circuit board in the ventilator while it is running. *Oxygen Tube Disconnection Alarm: Unplug the oxygen intake tube from the ventilator while the machine is on. *Mechanical Failure/Fatigue (# of cycles): Leave the machine running for extended periods of time (a few hours, days, weeks) and observe any inaccuracies.
- Ventilator Specifications that Have to be Achieved:
*RR: 6-40 breathes per minute (BPM) [Note: RR between 6-9 BPM are only for Assist Control] *TV: 200-800 mL (based on patient weight) *I:E: 1:2, best if adjustable between 1:1-1:4 * Assist Control based on Trigger Sensitivity: when a patient inspires, they can cause a dip of between 2-7 cm H2O, with respect to PEEP pressure *Maximum airway pressure limited to 40 cm H2O and must be continually monitored *Plateau pressure limited to a max 30 cm H2O *Passive mechanical blow-off valve fixed at 40 cm H2O is strongly recommended *Plateau pressure and PEEP readings required for clinician MIT Clinical *PEEP: 5-15 cm H2O (many patients need 10-15 cm H2O) *Alarms must be present for failure conditions and the switch to manual clinician override must be immediate *Room air for ventilation is fine for emergency scenarios *HEPA filtration on patients exhalation, or between vent and patient, is required due to COVID-19 being airborne
- Ideal Values that Should be Targeted for a Healthy Patient:
*PIP: 10-14 cm H2O *RR: 8-12 BPM
*PEEP: 3-10 cm H2O
*I:E: 1:2
*TV: 6-8 mL/kg
*O2 Concentration: 19.5-23.5%
*FiO2: 1.0 (100%)
* Mechanical Failure/Fatigue (# of cycles): ideally for as long as the patient requires the use of the ventilation (~ 2 weeks)
Regulations[edit | edit source]
- FDA's activities regarding medical devices: https://www.fda.gov/medical-devices
*COVID-19 activities: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-related-guidance-documents-industry-fda-staff-and-other-stakeholders
- Policy for ventilators: https://www.fda.gov/media/136318/download
- Emergency Use Authorization: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
* A ventilator would fall under other medical devices: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covidothermeddev
- FDA’s enforcement policy for certain modifications to ventilators and accessories and other respiratory devices during COVID-19: https://www.fda.gov/media/136318/download
- Importation of a medical device: https://content.govdelivery.com/bulletins/gd/USDHSCBP-282c648?wgt_ref=USDHSCBP_WIDGET_2?utm_source=csms.cbp.gov&utm_medium=csms.cbp.gov&utm_term=undefined&utm_content=undefined&utm_campaign=(not%20set)&gclid=undefined&dclid=undefined&GAID=1278996374.1567630590
- In regards to what kind of air should be used: https://www.fda.gov/media/136437/download
Safety Precautions[edit | edit source]
- Confirm that the wiring in the ventilator is correctly set-up (see wiring diagram)
- Make sure that the ventilator is properly connected to the artificial lung with the proper adapter size (see picture below)

- Confirm that the artificial lung is set-up correctly BEFORE turning the machine on (see manual below, pg.11)
https://www.michiganinstruments.com/wp-content/uploads/2018/11/TTL3OpsManualREV2017-05.pdf