Solar distillation

Solar distillation is the use of solar energy to evaporate water and collect its condensate within the same closed system. Unlike other forms of water purification it can turn salt or brackish water into fresh drinking water (e.g. desalination).
The structure that houses the process is known as a solar still and although the size, dimensions, materials, and configuration are varied, all rely on the simple procedure wherein an influent solution enters the system and the more volatile solvents leave in the effluent leaving behind the salty solute behind.[1]
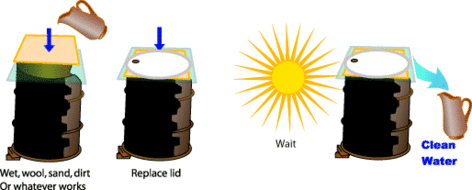
In any solar still, the basic layout is a collection device to capture rainwater. In most cases the collector is covered by a sheet of glass or transparent plastic, which allows solar radiation to pass through but not to escape. Water evaporated by the radiant solar heat then condenses on the cooler cover material. The condensed water is free from impurities, such as salts and heavy metals, as well as microbiological organisms, that might have been present in the intake water. The end result is a supply of fresh, clean water. Solar stills can efficiently produce drinking water from ditch water or cistern water, especially high-efficiency multiple effect humidification designs, which separate the evaporator(s) and condenser(s).
Solar distillation differs from other forms of desalination that are more energy-intensive, such as reverse osmosis or simply boiling water, due to its use of free energy.[2][3] If treatment of polluted water is required rather than desalination, slow sand filtration is a good option.
Types[edit | edit source]

From most sophisticated to least so, their three basic configurations are:
- Box-like
- Cone-shaped
- Pit type
The fundamental aspects of a solar still have gone unchanged since ancient times, the simplicity of the design is one of the solar still's chief benefits. However, there are many variations on the theme of the typical single slope/basin still and these can fall into one of two categories: active or passive. These labels classify the still by the method it uses to acquire the energy to drive the evaporation of the water. Passive solar stills are, of course, more conventional and have been the only ones discussed up to this point. Active stills, however, can obtain "waste" heat from a myriad of sources. A good insulator is necessary to reduce thermal losses and prolong the evaporation process even into the night.[4][5] Insulation that could be used include things like styrofoam with a polypropylene cover, or wool (which, can retain some of its insulation even when wet).[6][7]
Passive solar stills[edit | edit source]
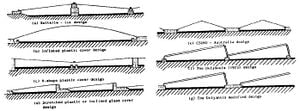
Conventional solar stills rely solely on the sun to distill water, however their complexity could still reach that of active stills, if not other more intricate desalination methods. Passive stills, then, vary widely due to this one constraint and can be further organized into sub-classes. Some common types of passive solar stills include:
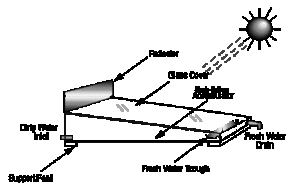
- Single-effect stills — Single-effect stills are the simplest and most common, since only one interface is necessary to convey the energy and collect the condensate. An example of a crucial design challenge in all solar stills is keeping the distiller airtight. If not airtight, efficiency drops severely. Often a shallow trough is used, painted black, and flooded. A slanted pane of glass covering, allowing condensed water vapor to slide down into an output channel. Expect 1 gallon per day per square meter of glass. Another approach is molded plastic (e.g. Watercone). This has the advantage that it is can be more easily made airtight, and mass production should make it affordable. Efficiencies of 25% are typical. Daily output as a function of solar irradiation is greatest in the early evening when the feed water is still hot but when outside temperatures are falling. Material selection is very important. The cover can be either glass or plastic. Glass is considered to be best for most long-term applications, whereas a plastic (such as polyethylene) can be used for short-term use. Sand concrete or waterproofed concrete are considered best for the basin of a long-life still if it is to be manufactured on-site, but for factory-manufactured stills, prefabricated ferro-concrete is a suitable material.
- Multi-effect stills — Multi-effect stills have two or more compartments. The condensing surface of the lower compartment is the floor of the upper compartment. The heat given off by the condensing vapor provides energy to vaporize the feed water above. Efficiency is therefore greater than for a single-basin still typically being 35% or more but the cost and complexity are correspondingly higher, require double the effort in regards to ensuring tight seals, and could be more to difficult to clean.[10] The way by which the water is stored for its time in the liquid phase can also contrast.
- Basin-type stills — Basin-type stills contain the water in an impervious material that is a component of the entire enclosure and are the most ubiqutious.
- Wick stills — In a wick still, the feed water flows slowly through a porous, radiation-absorbing pad (the wick). Two advantages are claimed over basin stills. First, the wick can be tilted so that the feed water presents a better angle to the sun (reducing reflection and presenting a large effective area). Second, less feed water is in the still at any time and so the water is heated more quickly and to a higher temperature. Wick stills use cloth-like materials that use capillary action to propagate the water through the system. When efficiency and effectiveness are key, wick stills out-produce basin stills due to the greater surface area of evaporation, lower energy cost to heat the water, and ability to create a much larger effective area for solar radiation to transfer energy into the water.[11] Some wick still designs can cost less than a basin still of the same output.
- Multi-wick stills — Multi-wick stills obviously play off of typical wick stills and much like the multi-effect premise from above, they greatly increase the productivity for increasing the influenced surface area exponentially.[12]
- Diffusion-type stills — Diffusion-type stills run with the ideas introduced by the mutli-effect & -wick stills and a further advancement to both. Perhaps, Tanaka & Nakatake best explain the design behind these efficient stills, "which consist of a series of closely spaced parallel partitions in contact with saline-soaked wicks, have great potential because of their high productivity and simplicity."[13]
- Greenhouse stills — Marry the concept of solar stills and greenhouses.
- Emergency still — To provide emergency drinking water on land, a very simple still can be made. It makes use of the moisture in the earth. All that is required is a plastic cover, a bowl or bucket, and a pebble.
Active solar stills[edit | edit source]
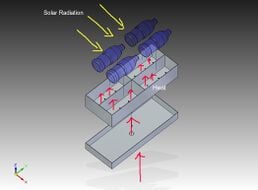
These distillers use additional heat sources to promote existing thermal processes.[14] The foundation of the design of these desalters has already been lain in the above section, so the sources involved with this branch of solar stills will be discussed with brevity:
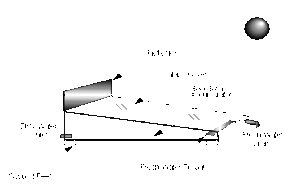
- Compound parabolic concentrators (CPCs)
- Flat plate collectors[15]
- Solar heater
- Novel waste heat — Waste heat can be used as an additional energy input, for example from an engine, the condenser of a refrigerator or a vehicle radiator.[16]
- Rainwater collection — By adding an external gutter, the still cover can be used for rainwater collection to supplement the solar still output.
Active stills add another element of complexity to the not so complex base design, but once again this alteration can promote faster, and larger quantities of freshwater generation.
Basic operating principle[edit | edit source]
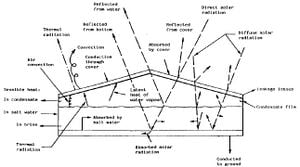
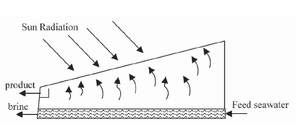
The main features of operation are the same for all solar stills. The incident solar radiation is transmitted through the glass or plastic cover and is absorbed as heat by a black surface in contact with the water to be distilled. The water is thus heated and gives off water vapor. The vapor condenses on the cover, which is at a lower temperature because it is in contact with the ambient air, and runs down into a gutter from where it is fed to a storage tank.
For high efficiency the solar still should maintain:
- High feed (undistilled) water temperature
- Large temperature difference between feed water and condensing surface
- Low vapor leakage
High feed water temperature can be achieved if:
- High proportion of incoming radiation is absorbed by the feed water as heat. Hence low absorption glazing and a good radiation absorbing surface are required
- Heat losses from the floor and walls are kept low
- Water is shallow so there is not so much to heat
Large temperature difference can be achieved if:
- Condensing surface absorbs little or none of the incoming radiation
- Condensing water dissipates heat which must be removed rapidly from the condensing surface by, for example, a second flow of water or air, or by condensing at night
Construction[edit | edit source]
Many different methods exist to build solar stills, the most rudimentary involves digging a hole and more the more complex coming off of a manufacturing line.
Common construction materials include:
- Insulation (usually under the basin)
- Sealants
- Piping and valves
- Facilities for storage
- Reflector to concentrate sunlight
- Structural components
- Wicking fabrics
- Blackened wood
- Black jute
- Black polyethylene
Locally available materials are generally preferred but many things, such as the sealants, might be necessary to find from foreign vendors.[12]
Since one of the main goals of solar distillation is to provide a clean source of water, proper disinfection after construction is crucial. Some less intensive methods of cleaning could be use of soap or laundry detergent. A glass cover is more advantageous with respect to maintenance than a plastic cover, due to the electrostatic properties of plastic that can make it a beacon for detritus.[17] The brine left over after thorough distillation can be harvested for sea salt, as it is now a valuable commodity in its own right.[18]
Single-basin still[edit | edit source]
Despite a proliferation of novel types, the single-basin still remains the only design proven in the field. At least 40 single-basin stills with areas of greater than 100m (and up to 9000m²) were built between 1957 and 1980. 27 had glass covers and 9 had plastic. 24 of the glass-covered stills are still operating in their original form, but only one plastic-covered unit is operational. Hundreds of smaller stills are operating, notably in Africa. The cost of pure water produced depends on the:
- Cost of making the still
- Cost of the land
- Life of the still
- Operating costs
- Cost of the feed water
- Discount rate adopted
- Amount of water produced
The cost of a solar still is normally UK £50-70/m². The price of land will normally be a small proportion of this in rural areas, but may be prohibitive in towns and cities. The life of a glass still is usually taken as 20 to 30 years but operating costs can be large especially to replace broken glass. Performance varies between tropical locations but not significantly. An average output of 2.5-3.0 1/m²/day is typical, that is, about 1m³/m²/year.
Applications[edit | edit source]
There is an important need for clean, pure drinking water in many developing countries. Often water sources are brackish (i.e. contain dissolved salts) and/or contain harmful bacteria and therefore cannot be used for drinking. In addition, there are many coastal locations where seawater is abundant but potable water is not available. Pure water is also useful for batteries and in hospitals or schools. Distillation is one of many processes that can be used for water purification. This requires an energy input, as heat, solar radiation can be the source of energy. In this process, water is evaporated, thus separating water vapor from dissolved matter, which is condensed as pure water.
Generally, solar stills are used in areas where piped or well water is impractical to obtain. Such areas include remote locations or locations where frequent power outages make pumps undependable. In such areas, solar stills can provide an alternate source of clean water. A major use of small solar stills is in developing countries where the technology to effectively distill large quantities of water on a commercial scale has not yet arrived. The drawback is that each individual still produces a relatively small amount of clean water.
Another application for solar stills is for outdoors back-country survival. Simple solar stills can be created by making use of basic camping gear and materials available in the natural environment. Stills for survival purposes would generally be of the relatively unsophisticated pit type, since they are the simplest to produce. One can extract moisture from the ground, but locally available moisture can be supplemented with water added inside or along the edges of the still. Where no water sources are readily available, urine or shredded vegetation can be used inside the pit. Whilst makeshift solar stills often do not provide enough water for long-term survival, they can prevent dehydration for short periods of time.
Many journals, researchers and others like them rely too much on the technical aspects of solar distillation to prove its worth.[19] In order to be socially sustainable, such technologies must:[19]
- Be accepted by the community
- Meet their water needs
- Be within their capacity to operate and maintain
The situation that exists today has not changed much from even 50 years ago. Energy and cost intensive technologies still champion over desalination in the modern world.[20] For this reason, many developing countries and communities, on large and small-scale levels resort to the status quo, when more appropriate solutions exist.[21]
Scaling and alternatives[edit | edit source]
Human beings need 1 or 2 litres of water a day to live. The minimum requirement for normal life in developing countries (which includes cooking, cleaning and washing clothes) is 20 litres per day (in the industrialized world 200 to 400 litres per day is typical). Yet some functions can be performed with salty water and a typical requirement for distilled water is 5 litres per person per day. Therefore 2m² of still are needed for each person served.
Solar stills should normally only be considered for removal of dissolved salts from water. If there is a choice between brackish ground water or polluted surface water, it will usually be cheaper to use a slow sand filter or other treatment device. If there is no fresh water then the main alternatives are desalination, transportation and rainwater collection.
Unlike other techniques of desalination, solar stills are more attractive the smaller the required output. The initial capital cost of stills is roughly proportional to capacity, whereas other methods have significant economies of scale. For the individual household, therefore, the solar still is most economic. For outputs of 1m³/day or more, reverse osmosis or electrodialysis should be considered as an alternative to solar stills. Much will depend on the availability and price of electrical power.
For outputs of 200m³/day or more, vapor compression or flash evaporation will normally be cheaper. The latter technology can have part of its energy requirement met by solar water heaters. In many parts of the world, fresh water is transported from another region or location by boat, train, truck or pipeline. The cost of water transported by vehicles is typically of the same order of magnitude as that produced by solar stills. A pipeline may be less expensive for very large quantities. Rainwater harvesting is an even simpler technique than solar distillation in areas where rain is not scarce, but requires a greater area and usually a larger storage tank. If ready-made collection surfaces exist (such as house roofs) these may provide a less expensive source for obtaining clean water.
Theory[edit | edit source]
A very common and, by far, the largest example of solar distillation is the natural water cycle that the Earth experiences. In "Understanding Solar Stills" it is said:[22]
It takes a lot of energy for water to vaporize. While a certain amount of energy is needed to raise the temperature of a kilogram of water from 0 to 100 Celcius (C), it takes five and one-half times that much to change it from water at 100°C to water vapor at 100°C. Practically all this energy, however, is given back when the water vapor condenses. This is the way we get fresh water in the clouds from the oceans, by solar distillation. All the fresh water on earth has been solar distilled.
The journey for a water molecule from the aqueous to gaseous phase is difficult. A tremendous factor will be the difference in temperature between the surface water and that of the interface, be it glass or plastic. Some relevant equations include:[23]
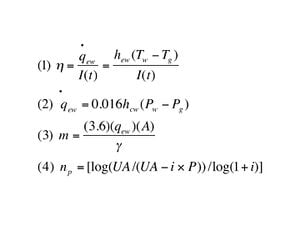
- Equation 1 describes the instantaneous thermal efficiency in relation to the evaporative heat transfer rate from the water surface to the glass cover the solar radiation intensity.
- Equation 2 represents the evaporative heat transfer rate from Eqn. (1) and its relationship to product of the convective heat transfer coefficient from the water surface to glass difference between the partial vapor pressure of water and gas.
- Equation 3 is the equation for determining the monthly output of distillate.
- Equation 4 was developed to describe the pay back period, np as a function of the Unacost, or the uniform end-of-year annual amount with P being the initial cost and i the interest rate.
The energy required to evaporate water is the latent heat of vaporisation of water. This has a value of 2260 kilojoules per kilogram (kJ/kg). This means that to produce 1 litre (e.g. 1kg since the density of water is 1kg/litre) of pure water by distilling brackish water requires a heat input of 2260kJ. This does not allow for the efficiency of the heating method, which will be less than 100%, or for any recovery of latent heat that is rejected when the water vapour is condensed.
It should be noted that, although 2260kJ/kg is required to evaporate water, to pump a kg of water through 20m head requires only 0.2kJ/kg. Distillation is therefore normally considered only where there is no local source of fresh water that can be easily pumped or lifted.
An approximate method of estimating the output of a solar still is given by:
- Q = (E x G x A) / 2.3
where:
- Q = daily output of distilled water (litres/day)
- E = overall efficiency
- G = daily global solar irradiation (MJ/m²)
- A = aperture area of the still ie, the plan areas for a simple basin still (²)
In a typical country the average, daily, global solar irradiation is typically 18.0 MJ/m² (5 kWh/m²). A simple basin still operates at an overall efficiency of about 30%. Hence the output per square metre of area is:
- daily output = (0.30 x 18.0 x 1) / 2.3 = 2.3 litres (per square metre)
The yearly output of a solar still is often therefore referred to as approximately one cubic metre per square metre.
History[edit | edit source]
Solar water distillation is a solar technology with a very long history and installations were built over 2000 years ago, although to produce salt rather than drinking water. Documented use of solar stills began in the sixteenth century. An early large-scale solar still was built in 1872 to supply a mining community in Chile with drinking water. Mass production occurred for the first time during the Second World War when 200,000 inflatable plastic stills were made to be kept in life-crafts for the US Navy.

Solar stills have been used for hundreds of years. The earliest known examples date to 1551 when Arab alchemists used such stills. In 1882 Charles Wilson invented the first modern conventional still — a massive solar still plant which was used to supply fresh water to a mining community in northern Chile. Today hundreds of solar still plants and thousands of individual solar stills have been built around the world.
The earliest onset of solar energy use to desalinate water is widely accredited to Aristotle during the fourth century B.C.E.[24][25][26][22] Earlier attributions reference the Bible & Moses' use of a piece of wood to remove the "bitterness" from water (Exodus 15:25, English Standard Version). The first documented account of solar distillation use for desalination was by Giovani Batista Della Porta in 1958.[24] However, no solar distillation publication of any repute leaves out the Father of solar distillation, Carlos Wilson, the creator of the first modern sun-powered desalination plant, built in Las Salinas (The Salts), Chile in 1872.[24][22][27][28][29][30] This desalination plant, "can be considered to be the first industrial installation for exploitation of solar energy."[30] The Las Salinas plant was envisioned to take advantage of the nearby saltpeter mining effluent to supply the miners and their families freshwater.[24] The facility was quite large for its time and now:[24]
The plant was constructed of wood and timber framework covered with one sheet of glass. It consisted of 64 bays having a total surface area of 4450 m2 and a total land surface area of 7896 m2. It produced 22.70 m3 of fresh water per day. The plant was in operation for about 40 years until the mines were exhausted.
Interest in solar distillation wavered for some time, until historical events prompted further research and development. World War II was a great catalyst for the Massachusetts Institute of Technology to develop appropriate solar stills for use in more remote areas of the world during emergencies. These small solar stills were made to float on and collect saltwater to desalt as they floated alongside life-boats and rafts.[24] More significant studies into solar distillation were carried out by the Office of Saline Water, a sector the US government, in 1952. Many experiments were performed on different conceptualizations of the solar still, including multiple-effect basins and the application of condensers.[24] This trend ended near the early 70's with the advent of more lucrative desalination techniques like the aforementioned reverse osmosis or multi-stage flash, a technique that involves a series of stages where evaporation relies on lowering the pressure of each stage to lower the boiling or "flashing" point of the water.[31][32] Today, renewed enthusiasm for solar distillation comes from individuals, communities, and organizations seeking an appropriate technology that is cheap, simple, and conceivable in rural settings.[17]
Related projects[edit | edit source]
See also[edit | edit source]
- Understanding Solar Stills (VITA publication)
- Compound parabolic concentrators
- Solar distillation TB
External links[edit | edit source]
- Wikipedia:Solar still
- Wikipedia:Water pyramid - Commercial solar still
- PV-based solar stills: a more efficient design - Separation of the 2 chambers can be better done
- Aquaver WTS-40 - Can use thermal energy/heat from the sun
- Fresh Water with 3D Printed Water Distiller
Further reading[edit | edit source]
- Malik A S et. al. (1982) Solar Distillation, Pergamon Press - Provides a comprehensive technical text
- Developing Appropriate Technologies in Peru (1988) Waterlines Journal, Vol 7, No 2.
References[edit | edit source]
- ↑ (2008). Desalination, a national perspective. National Research Council of the National Academies.
- ↑ Abu-Arabi, M. (2007). Status and prospects for solar desalination in the MENA region. Solar Desalination for the 21 st Century, 163-178.
- ↑ Paton, C., & Davies, P. (2006). The seawater greenhouse cooling, fresh water and fresh produce from seawater. In The 2nd International Conference on Water Resources in Arid Environments, Riyadh.
- ↑ Löf, G. O. (1961). Fundamental problems in solar distillation. Proceedings of the National Academy of Sciences of the United States of America, 47(8), 1279.
- ↑ Goosen, M. F., Sablani, S. S., Shayya, W. H., Paton, C., & Al-Hinai, H. (2000). Thermodynamic and economic considerations in solar desalination. Desalination, 129(1), 63-89.
- ↑ Ihalawela, P. H. C. A., & Careem, M. A. (2007). A cheap automatic solar water distiller. In Proceedings of the technical sessions (Vol. 23, pp. 41-45).
- ↑ BACHA, H., Maalej, A. Y., & DHIA, H. B. (2007). A Methodology to Predict Operation of a Solar Powered Desalination Unit. Solar Desalination for the 21 st Century, 69-82.
- ↑ Ettouney, H., & Rizzuti, L. (2007). SOLAR DESALINATION: A CHALLENGE FOR SUSTAINABLE FRESH WATER IN THE 21 ST CENTURY. Solar Desalination for the 21 st Century, 1-18.
- ↑ Paton, C., & Davies, P. (2006). The seawater greenhouse cooling, fresh water and fresh produce from seawater. In The 2nd International Conference on Water Resources in Arid Environments, Riyadh.
- ↑ Blanco, J., & Alarcón, D. (2007). The PSA experience on solar desalination: technology development and research activities. Solar Desalination for the 21 st Century, 195-206.
- ↑ Noble, Neil (2012). Solar Distillation. Retrieved from http://web.archive.org/web/20140608080946/http://practicalaction.org:80/solar-distillation-1
- ↑ 12.0 12.1 Velmurugan, V., & Srithar, K. (2011). Performance analysis of solar stills based on various factors affecting the productivity—A review. Renewable and Sustainable Energy Reviews, 15(2), 1294-1304.
- ↑ Tanaka, H., & Nakatake, Y. (2007). Outdoor experiments of a vertical diffusion solar still coupled with a flat plate reflector. Desalination, 214(1), 70-82.
- ↑ AYBAR, H. (2007). A review of desalination by solar still. Solar Desalination for the 21 st Century, 207-214.
- ↑ Kabeel, A. E., & El-Agouz, S. A. (2011). Review of researches and developments on solar stills. Desalination, 276(1), 1-12.
- ↑ Mandaville, J. (1972). Some Experiments with Solar Ground Stills in Eastern Arabia. Geographical Journal, 64-66.
- ↑ 17.0 17.1 Eibling, J. A., Talbert, S. G., & Löf, G. O. G. (1971). Solar stills for community use—digest of technology. Solar energy, 13(2), 263-276.
- ↑ KOPSCH, O. (2007). SOLAR STILLS: 10 YEARS OF PRACTICAL EXPERIENCE IN COMMERCIALISING SOLAR STILLS WORLDWIDE. Solar Desalination for the 21 st Century, 239-246.
- ↑ 19.0 19.1 Werner, M., & Schäfer, A. I. (2007). Social aspects of a solar-powered desalination unit for remote Australian communities. Desalination, 203(1), 375-393.
- ↑ Chaibi, M. T. (2000). An overview of solar desalination for domestic and agriculture water needs in remote arid areas. Desalination, 127(2), 119-133.
- ↑ Bloemer, J. W., Eibling, J. A., Irwin, J. R., & Löf, G. O. (1965). A practical basin-type solar still. Solar Energy, 9(4), 197-200.
- ↑ 22.0 22.1 22.2 Gordes, J., & McCracken, H. (1985). Understanding Solar Stills. Volunteers in Technical Assistance (VITA).
- ↑ Medugu, D. W., & Ndatuwong, L. G. (2009). Theoretical analysis of water distillation using solar still. International Journal of Physical Sciences, 4(11), 705-712.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 24.8 Delyannis, E. (2003). Historic background of desalination and renewable energies. Solar Energy, 75(5), 357-366.
- ↑ Tiwari, G. N., Singh, H. N., & Tripathi, R. (2003). Present status of solar distillation. Solar Energy, 75(5), 367-373.
- ↑ Velmurugan, V., & Srithar, K. (2011). Performance analysis of solar stills based on various factors affecting the productivity—A review. Renewable and Sustainable Energy Reviews, 15(2), 1294-1304.
- ↑ Al-Hayeka, I., & Badran, O. O. (2004). The effect of using different designs of solar stills on water distillation. Desalination, 169(2), 121-127.
- ↑ Goosen, M. F., Sablani, S. S., Shayya, W. H., Paton, C., & Al-Hinai, H. (2000). Thermodynamic and economic considerations in solar desalination. Desalination, 129(1), 63-89.
- ↑ Bouchekima, B. (2003). A small solar desalination plant for the production of drinking water in remote arid areas of southern Algeria. Desalination, 159(2), 197-204.
- ↑ 30.0 30.1 Hirschmann, J. R. (1975). Solar distillation in Chile. Desalination, 17(1), 31-67.
- ↑ El-Dessouky, H. T., Ettouney, H. M., & Al-Roumi, Y. (1999). Multi-stage flash desalination: present and future outlook. Chemical Engineering Journal, 73(2), 173-190.
- ↑ Fath, H. E. (1998). Solar distillation: a promising alternative for water provision with free energy, simple technology and a clean environment. Desalination, 116(1), 45-56.